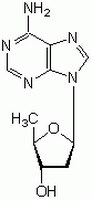
288104 Sigma-Aldrich2ʹ,5ʹ-Dideoxyadenosine - CAS 6698-26-6 - Calbiochem
Cell-permeable, non-competitive adenylate cyclase inhibitor (IC₅₀ = 3 µM), that binds to the adenosine P1 binding site.
More>> Cell-permeable, non-competitive adenylate cyclase inhibitor (IC₅₀ = 3 µM), that binds to the adenosine P1 binding site. Less<<Synonymes: 2ʹ,5ʹ-dd-Ado
Produits recommandés
Aperçu
| Replacement Information |
|---|
Tableau de caractéristiques principal
| CAS # | Empirical Formula |
|---|---|
| 6698-26-6 | C₁₀H₁₃N₅O₂ |
Products
| Référence | Conditionnement | Qté | |
|---|---|---|---|
| 288104-1MG | Ampoule plast. | 1 mg |
| Product Information | |
|---|---|
| CAS number | 6698-26-6 |
| ATP Competitive | N |
| Form | White solid |
| Hill Formula | C₁₀H₁₃N₅O₂ |
| Chemical formula | C₁₀H₁₃N₅O₂ |
| Reversible | N |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | adenylate cyclase |
| Primary Target IC<sub>50</sub> | 3 µM against adenylate cyclase |
| Purity | ≥98% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information |
|---|
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Référence | GTIN |
| 288104-1MG | 04055977197914 |
Documentation
2ʹ,5ʹ-Dideoxyadenosine - CAS 6698-26-6 - Calbiochem FDS
| Titre |
|---|
2ʹ,5ʹ-Dideoxyadenosine - CAS 6698-26-6 - Calbiochem Certificats d'analyse
| Titre | Numéro de lot |
|---|---|
| 288104 |
Références bibliographiques
| Aperçu de la référence bibliographique |
|---|
| Ibrahimi, A., et al. 1999. Am. J. Physiol. 276, C487. Hartman, M., and Schrader, J. 1995. J. Mol. Cell. Cardiol. 25, 331. Bushfield, M., et al. 1990. Mol. Pharmacol. 38, 848. Reid, I.R., et al. 1990. Am. J. Physiol. 258, E708. Legrand, A.B., et al. 1990. Biochem. Pharmacol. 40, 1103. Johnson, R.A., et al. 1989. Mol. Pharmacol. 35, 681. Holgate, S.T., et al. 1980. Proc. Natl. Acad. Sci. USA 77, 6800. |
Brochure
| Titre |
|---|
| Activators and Inhibitors of Adenylate Cyclase Technical Bulletin |