565790 Sigma-Aldrichγ-Secretase Inhibitor XXI, Compound E - CAS 209986-17-4 - Calbiochem
This g-secretase inhibitor, CAS 209986-17-4, is a cell-permeable, potent, selective, non-transition-state analog inhibitor of γ-secretase and Notch processing. Lowers Aβ levels in APP transgenic mice
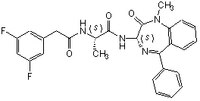
More>> This g-secretase inhibitor, CAS 209986-17-4, is a cell-permeable, potent, selective, non-transition-state analog inhibitor of γ-secretase and Notch processing. Lowers Aβ levels in APP transgenic mice Less<<Synonymes: (S,S)- 2-[2-(3,5-Difluorophenyl)-acetylamino]-N-(1-methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benzo[e][1,4]diazepin-3-yl)-propionamide, Compound E
Produits recommandés
Aperçu
Tableau de caractéristiques principal
| CAS # | Empirical Formula |
|---|---|
| 209986-17-4 | C₂₇H₂₄F₂N₄O₃ |
Products
| Référence | Conditionnement | Qté | |
|---|---|---|---|
| 565790-1MG | Flacon en verre | 1 mg | |
| 565790-500UG | Ampoule plast. | 500 μg |
| Product Information | |
|---|---|
| CAS number | 209986-17-4 |
| ATP Competitive | N |
| Form | White solid |
| Hill Formula | C₂₇H₂₄F₂N₄O₃ |
| Chemical formula | C₂₇H₂₄F₂N₄O₃ |
| Reversible | N |
| Structure formula Image | |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Global Trade Item Number | |
|---|---|
| Référence | GTIN |
| 565790-1MG | 04055977191851 |
| 565790-500UG | 04055977191868 |