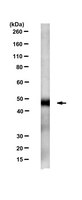
Human IFIT3 Modulates IFIT1 RNA Binding Specificity and Protein Stability.
Johnson, B; VanBlargan, LA; Xu, W; White, JP; Shan, C; Shi, PY; Zhang, R; Adhikari, J; Gross, ML; Leung, DW; Diamond, MS; Amarasinghe, GK
Immunity
48
487-499.e5
2018
Show Abstract
Although interferon-induced proteins with tetratricopeptide repeats (IFIT proteins) inhibit infection of many viruses by recognizing their RNA, the regulatory mechanisms involved remain unclear. Here we report a crystal structure of cap 0 (m7GpppN) RNA bound to human IFIT1 in complex with the C-terminal domain of human IFIT3. Structural, biochemical, and genetic studies suggest that IFIT3 binding to IFIT1 has dual regulatory functions: (1) extending the half-life of IFIT1 and thereby increasing its steady-state amounts in cells; and (2) allosterically regulating the IFIT1 RNA-binding channel, thereby enhancing the specificity of recognition for cap 0 but not cap 1 (m7GpppNm) or 5'-ppp RNA. Mouse Ifit3 lacks this key C-terminal domain and does not bind mouse Ifit1. The IFIT3 interaction with IFIT1 is important for restricting infection of viruses lacking 2'-O methylation in their RNA cap structures. Our experiments establish differences in the regulation of IFIT1 orthologs and define targets for modulation of human IFIT protein activity. | 29525521
 |
A therapeutic antibody against west nile virus neutralizes infection by blocking fusion within endosomes.
Thompson, BS; Moesker, B; Smit, JM; Wilschut, J; Diamond, MS; Fremont, DH
PLoS Pathog
5
e1000453
2009
Show Abstract
Defining the precise cellular mechanisms of neutralization by potently inhibitory antibodies is important for understanding how the immune system successfully limits viral infections. We recently described a potently inhibitory monoclonal antibody (MAb E16) against the envelope (E) protein of West Nile virus (WNV) that neutralizes infection even after virus has spread to the central nervous system. Herein, we define its mechanism of inhibition. E16 blocks infection primarily at a post-attachment step as antibody-opsonized WNV enters permissive cells but cannot escape from endocytic compartments. These cellular experiments suggest that E16 blocks the acid-catalyzed fusion step that is required for nucleocapsid entry into the cytoplasm. Indeed, E16 directly inhibits fusion of WNV with liposomes. Additionally, low-pH exposure of E16-WNV complexes in the absence of target membranes did not fully inactivate infectious virus, further suggesting that E16 prevents a structural transition required for fusion. Thus, a strongly neutralizing anti-WNV MAb with therapeutic potential is potently inhibitory because it blocks viral fusion and thereby promotes clearance by delivering virus to the lysosome for destruction. | 19478866
 |
Antibody recognition and neutralization determinants on domains I and II of West Nile Virus envelope protein.
Oliphant, T; Nybakken, GE; Engle, M; Xu, Q; Nelson, CA; Sukupolvi-Petty, S; Marri, A; Lachmi, BE; Olshevsky, U; Fremont, DH; Pierson, TC; Diamond, MS
J Virol
80
12149-59
2006
Show Abstract
Previous studies have demonstrated that monoclonal antibodies (MAbs) against an epitope on the lateral surface of domain III (DIII) of the West Nile virus (WNV) envelope (E) strongly protect against infection in animals. Herein, we observed significantly less efficient neutralization by 89 MAbs that recognized domain I (DI) or II (DII) of WNV E protein. Moreover, in cells expressing Fc gamma receptors, many of the DI- and DII-specific MAbs enhanced infection over a broad range of concentrations. Using yeast surface display of E protein variants, we identified 25 E protein residues to be critical for recognition by DI- or DII-specific neutralizing MAbs. These residues cluster into six novel and one previously characterized epitope located on the lateral ridge of DI, the linker region between DI and DIII, the hinge interface between DI and DII, and the lateral ridge, central interface, dimer interface, and fusion loop of DII. Approximately 45% of DI-DII-specific MAbs showed reduced binding with mutations in the highly conserved fusion loop in DII: 85% of these (34 of 40) cross-reacted with the distantly related dengue virus (DENV). In contrast, MAbs that bound the other neutralizing epitopes in DI and DII showed no apparent cross-reactivity with DENV E protein. Surprisingly, several of the neutralizing epitopes were located in solvent-inaccessible positions in the context of the available pseudoatomic model of WNV. Nonetheless, DI and DII MAbs protect against WNV infection in mice, albeit with lower efficiency than DIII-specific neutralizing MAbs. | 17035317
 |