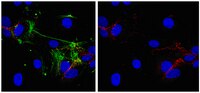
The assessment of CD146-based cell sorting and telomere length analysis for establishing the identity of mesenchymal stem cells in human umbilical cord.
Kouroupis, D; Churchman, SM; McGonagle, D; Jones, EA
F1000Research
3
126
2014
Show Abstract
Adult stem cells are characterised by longer telomeres compared to mature cells from the same tissue. In this study, candidate CD146 (+) umbilical cord (UC) mesenchymal stem cells (MSCs) were purified by cell sorting from UC tissue digests and their telomere lengths were measured in comparison to donor-matched CD146-negative fraction. UC tissue fragments were enzymatically treated with collagenase and the cells were used for cell sorting, colony-forming fibroblast (CFU-F) assay or for long-term MSC cultivation. Telomere lengths were measured by qPCR in both culture-expanded MSCs and candidate native UC MSCs. Immunohistochemistry was undertaken to study the topography of CD146 (+) cells. Culture-expanded UC MSCs had a stable expression of CD73, CD90 and CD105, whereas CD146 declined in later passages which correlated with the shortening of telomeres in the same cultures. In five out of seven donors, telomeres in candidate native UC MSCs (CD45 (-)CD235α (-)CD31 (-)CD146 (+)) were longer compared to donor-matched CD146 (-) population (CD45 (-)CD235α (-)CD31 (-)CD146 (-)). The frequency of CD45 (-)CD235α (-)CD31 (-)CD146 (+) cells measured by flow cytometry was ~1000-fold above that of CFU-Fs (means 10.4% and 0.01%, respectively). CD146 (+) cells were also abundant in situ having a broad topography including high levels of positivity in muscle areas in addition to vessels. Although qPCR-based telomere length analysis in sorted populations could be limited in its sensitivity, very high frequency of CD146 (+) cells in UC tissue suggests that CD146 expression alone is unlikely to be sufficient to identify and purify native MSCs from the UC tissue. | Immunohistochemistry | 25232467
 |
Induced in vitro differentiation of neural-like cells from human exfoliated deciduous teeth-derived stem cells.
Nourbakhsh, N; Soleimani, M; Taghipour, Z; Karbalaie, K; Mousavi, SB; Talebi, A; Nadali, F; Tanhaei, S; Kiyani, GA; Nematollahi, M; Rabiei, F; Mardani, M; Bahramiyan, H; Torabinejad, M; Nasr-Esfahani, MH; Baharvand, H
The International journal of developmental biology
55
189-95
2011
Show Abstract
Stem cells from human exfoliated deciduous teeth (SHED) are highly proliferative, clonogenic and multipotent stem cells with a neural crest cell origin. Additionally, they can be collected with minimal invasiveness in comparison with other sources of mesenchymal stem cells (MSCs). Therefore, SHED could be a desirable option for potential therapeutic applications. In this study, SHEDs were established from enzyme-disaggregated deciduous dental pulp obtained from 6 to 9 year-old children. The cells had typical fibroblastoid morphology and expressed antigens characteristic of MSCs, STRO1, CD146, CD45, CD90, CD106 and CD166, but not the hematopoietic and endothelial markers, CD34 and CD31, as assessed by FACS analysis. Differentiation assessment revealed a strong osteogenic and adipogenic potential of SHEDs. In order to further evaluate the in vitro differentiation potential of SHED into neural cells, a simple short time growth factor-mediated induction was used. Immunofluorescence staining and flow cytometric analysis revealed that SHED rapidly expressed nestin and b-III tubulin, and later expressed intermediate neural markers. In addition, the intensity and percentages of nestin and b-III tubulin and mature neural markers (PSA-NCAM, NeuN, Tau, TH, or GFAP) increased significantly following treatment. Moreover, RT-PCR and Western blot analyses showed that the neural markers were strongly up-regulated after induction. In conclusion, these results provide evidence that SHED can differentiate into neural cells by the expression of a comprehensive set of genes and proteins that define neural-like cells in vitro. SHED cells might be considered as new candidates for the autologous transplantation of a wide variety of neurological diseases and neurotraumatic injuries. | | 21671222
 |
Global array-based transcriptomics from minimal input RNA utilising an optimal RNA isolation process combined with SPIA cDNA probes.
Kennedy, L; Pauriah, M; Godfrey, V; Howie, J; Dennis, H; Crowther, D; Struthers, A; Goddard, C; Feuerstein, G; Lang, C; Miele, G
PloS one
6
e17625
2011
Show Abstract
Technical advances in the collection of clinical material, such as laser capture microdissection and cell sorting, provide the advantage of yielding more refined and homogenous populations of cells. However, these attractive advantages are counter balanced by the significant difficulty in obtaining adequate nucleic acid yields to allow transcriptomic analyses. Established technologies are available to carry out global transcriptomics using nanograms of input RNA, however, many clinical samples of low cell content would be expected to yield RNA within the picogram range. To fully exploit these clinical samples the challenge of isolating adequate RNA yield directly and generating sufficient microarray probes for global transcriptional profiling from this low level RNA input has been addressed in the current report. We have established an optimised RNA isolation workflow specifically designed to yield maximal RNA from minimal cell numbers. This procedure obtained RNA yield sufficient for carrying out global transcriptional profiling from vascular endothelial cell biopsies, clinical material not previously amenable to global transcriptomic approaches. In addition, by assessing the performance of two linear isothermal probe generation methods at decreasing input levels of good quality RNA we demonstrated robust detection of a class of low abundance transcripts (GPCRs) at input levels within the picogram range, a lower level of RNA input (50 pg) than previously reported for global transcriptional profiling and report the ability to interrogate the transcriptome from only 10 pg of input RNA. By exploiting an optimal RNA isolation workflow specifically for samples of low cell content, and linear isothermal RNA amplification methods for low level RNA input we were able to perform global transcriptomics on valuable and potentially informative clinically derived vascular endothelial biopsies here for the first time. These workflows provide the ability to robustly exploit ever more common clinical samples yielding extremely low cell numbers and RNA yields for global transcriptomics. Full Text Article | | 21445340
 |
Estrogen-mediated endothelial progenitor cell biology and kinetics for physiological postnatal vasculogenesis.
Masuda, H; Kalka, C; Takahashi, T; Yoshida, M; Wada, M; Kobori, M; Itoh, R; Iwaguro, H; Eguchi, M; Iwami, Y; Tanaka, R; Nakagawa, Y; Sugimoto, A; Ninomiya, S; Hayashi, S; Kato, S; Asahara, T
Circulation research
101
598-606
2007
Show Abstract
Estrogen has been demonstrated to promote therapeutic reendothelialization after vascular injury by bone marrow (BM)-derived endothelial progenitor cell (EPC) mobilization and phenotypic modulation. We investigated the primary hypothesis that estrogen regulates physiological postnatal vasculogenesis by modulating bioactivity of BM-derived EPCs through the estrogen receptor (ER), in cyclic hormonally regulated endometrial neovascularization. Cultured human EPCs from peripheral blood mononuclear cells (PB-MNCs) disclosed consistent gene expression of ER alpha as well as downregulated gene expressions of ER beta. Under the physiological concentrations of estrogen (17beta-estradiol, E2), proliferation and migration were stimulated, whereas apoptosis was inhibited on day 7 cultured EPCs. These estrogen-induced activities were blocked by the receptor antagonist, ICI182,780 (ICI). In BM transplanted (BMT) mice with ovariectomy (OVX) from transgenic mice overexpressing beta-galactosidase (lacZ) regulated by an endothelial specific Tie-2 promoter (Tie-2/lacZ/BM), the uterus demonstrated a significant increase in BM-derived EPCs (lacZ expressing cells) incorporated into neovasculatures detected by CD31 immunohistochemistry after E2 administration. The BM-derived EPCs that were incorporated into the uterus dominantly expressed ER alpha, rather than ER beta in BMT mice from BM of transgenic mice overexpressing EGFP regulated by Tie-2 promoter with OVX (Tie-2/EGFP/BMT/OVX) by ERs fluorescence immunohistochemistry. An in vitro assay for colony forming activity as well as flow cytometry for CD133, CD34, KDR, and VE-cadherin, using human PB-MNCs at 5 stages of the female menstrual-cycle (early-proliferative, pre-ovulatory, post-ovulatory, mid-luteal, late-luteal), revealed cycle-specific regulation of EPC kinetics. These findings demonstrate that physiological postnatal vasculogenesis involves cyclic, E2-regulated bioactivity of BM-derived EPCs, predominantly through the ER alpha. | | 17656679
 |
Differential distribution of stem cells in the auditory and vestibular organs of the inner ear.
Oshima, K; Grimm, CM; Corrales, CE; Senn, P; Martinez Monedero, R; Géléoc, GS; Edge, A; Holt, JR; Heller, S
Journal of the Association for Research in Otolaryngology : JARO
8
18-31
2007
Show Abstract
The adult mammalian cochlea lacks regenerative capacity, which is the main reason for the permanence of hearing loss. Vestibular organs, in contrast, replace a small number of lost hair cells. The reason for this difference is unknown. In this work we show isolation of sphere-forming stem cells from the early postnatal organ of Corti, vestibular sensory epithelia, the spiral ganglion, and the stria vascularis. Organ of Corti and vestibular sensory epithelial stem cells give rise to cells that express multiple hair cell markers and express functional ion channels reminiscent of nascent hair cells. Spiral ganglion stem cells display features of neural stem cells and can give rise to neurons and glial cell types. We found that the ability for sphere formation in the mouse cochlea decreases about 100-fold during the second and third postnatal weeks; this decrease is substantially faster than the reduction of stem cells in vestibular organs, which maintain their stem cell population also at older ages. Coincidentally, the relative expression of developmental and progenitor cell markers in the cochlea decreases during the first 3 postnatal weeks, which is in sharp contrast to the vestibular system, where expression of progenitor cell markers remains constant or even increases during this period. Our findings indicate that the lack of regenerative capacity in the adult mammalian cochlea is either a result of an early postnatal loss of stem cells or diminishment of stem cell features of maturing cochlear cells. Full Text Article | | 17171473
 |
A SAGE-based comparison between glomerular and aortic endothelial cells.
Sengoelge, G; Luo, W; Fine, D; Perschl, AM; Fierlbeck, W; Haririan, A; Sorensson, J; Rehman, TU; Hauser, P; Trevick, JS; Kulak, SC; Wegner, B; Ballermann, BJ
American journal of physiology. Renal physiology
288
F1290-300
2005
Show Abstract
Endothelial cells have many characteristics in common, but significant morphological and functional differences exist between endothelial cells from different anatomic sites. The specific glomerular endothelial (GEn) cell transcript repertoire is unknown. We sought to determine whether endothelial cells derived from bovine glomeruli display a distinct transcriptional profile compared with bovine aortic endothelium (BAE) under identical conditions. Serial analysis of gene expression (SAGE), which includes known and unknown transcripts, was used to make the comparison. The GEn and BAE SAGE libraries contain 36,844 and 26,452 total tag sequences, respectively. Among 6,524 unique tag sequences represented at least 2 times in the 2 libraries, 2,094 (32%) were matched to well-characterized bovine cDNA sequences (358 tags) or expressed sequence tags (EST). Identification of the human homolog was achieved for 1,035 of these tags. Forty-two tags were differentially expressed in GEn. For 25 of these, the bovine cDNA or EST, and for 17 the human homolog was identified. Among all transcripts with a known bovine and human tag, seven were expressed at levels more than 10-fold higher in cultured GEn cells compared with all other SAGE libraries. The transcript "DKFZp564B076" was localized by in situ hybridization to glomerular endothelium in vivo and was shown by real-time RT-PCR to be highly abundant in glomeruli compared with aortic intima. This work supports the concept that differences in the transcriptional profile of endothelial cells from distinct origins are observed under otherwise equivalent conditions. Furthermore, we have identified the first known transcript predominant in glomerular endothelium in vivo. | | 15657302
 |
Cell fusion-independent differentiation of neural stem cells to the endothelial lineage.
Wurmser, Andrew E, et al.
Nature, 430: 350-6 (2004)
2004
| | 15254537
 |
Origin of endothelial progenitors in human postnatal bone marrow.
Reyes, M; Dudek, A; Jahagirdar, B; Koodie, L; Marker, PH; Verfaillie, CM
The Journal of clinical investigation
109
337-46
2002
Show Abstract
This study demonstrates that a CD34(-), vascular endothelial cadherin(-) (VE-cadherin(-)), AC133(+), and fetal liver kinase(+) (Flk1(+)) multipotent adult progenitor cell (MAPC) that copurifies with mesenchymal stem cells from postnatal human bone marrow (BM) is a progenitor for angioblasts. In vitro, MAPCs cultured with VEGF differentiate into CD34(+), VE-cadherin(+), Flk1(+) cells - a phenotype that would be expected for angioblasts. They subsequently differentiate into cells that express endothelial markers, function in vitro as mature endothelial cells, and contribute to neoangiogenesis in vivo during tumor angiogenesis and wound healing. This in vitro model of preangioblast-to-endothelium differentiation should prove very useful in studying commitment to the angioblast and beyond. In vivo, MAPCs can differentiate in response to local cues into endothelial cells that contribute to neoangiogenesis in tumors. Because MAPCs can be expanded in culture without obvious senescence for more than 80 population doublings, they may be an important source of endothelial cells for cellular pro- or anti-angiogenic therapies. | | 11827993
 |
HIV-1-Tat protein activates phosphatidylinositol 3-kinase/ AKT-dependent survival pathways in Kaposi's sarcoma cells.
Deregibus, Maria Chiara, et al.
J. Biol. Chem., 277: 25195-202 (2002)
2002
Show Abstract
In this study we found that Tat protected vincristine-treated Kaposi's sarcoma cells from apoptosis and from down-regulation of several anti-apoptotic genes such as AKT-1, AKT-2, BCL2, BCL-XL, and insulin-like growth factor I and induced the de novo expression of the interleukin-3 gene. Moreover, we found that Tat enhanced phosphorylation of AKT and BAD proteins. The inhibition of phosphatidylinositol 3-kinase with two unrelated pharmacological inhibitors, wortmannin and LY294002, abrogated both the anti-apoptotic effect and the phosphorylation of AKT induced by Tat. After treatment with Tat, the AKT enzymatic activity showed a biphasic increase: an early activation (15 min), independent from protein synthesis; and a delayed activation (24 h), which was significantly decreased upon blockage of protein synthesis. Experiments with a function blocking anti-vascular endothelial cell growth factor receptor-2 antibody suggested that both the early and delayed AKT activation and the protection from apoptosis were triggered by the interaction of Tat with vascular endothelial cell growth factor receptor-2. Moreover, experiments with function-blocking antibodies directed against insulin-like growth factor I/insulin-like growth factor I receptor or interleukin-3 indicated their involvement in the delayed activation of AKT and their contribution to the anti-apoptotic effect of Tat on vincristine-treated Kaposi's sarcoma cells. | Immunoprecipitation | 11994280
 |
Glomerulosclerosis is transmitted by bone marrow-derived mesangial cell progenitors.
Cornacchia, F; Fornoni, A; Plati, AR; Thomas, A; Wang, Y; Inverardi, L; Striker, LJ; Striker, GE
The Journal of clinical investigation
108
1649-56
2001
Show Abstract
We found that ROP Os/+ (Os/+) mice had diffuse glomerulosclerosis and glomerular hypertrophy and that their mesangial cells (the vascular smooth muscle cells of the glomerulus) displayed an apparent sclerosing phenotype. Since mesangial cells are the major source of scar tissue in glomerulosclerosis, we postulated that the sclerosis phenotype was carried by mesangial cell progenitors and that this phenotype could be derived from the bone marrow (BM). Therefore, we transplanted BM from Os/+ mice into congenic ROP +/+ mice (+/+ mice), which have normal glomeruli. We found that glomeruli of +/+ recipients of Os/+ marrow contained the Os/+ genotype, were hypertrophied, and contained increased extracellular matrix. Clones of recipient glomerular mesangial cells with the donor genotype were found in all +/+ recipients that developed mesangial sclerosis and glomerular hypertrophy, whereas +/+ recipients of +/+ BM had normal glomeruli. Thus, the sclerotic (Os/+) or normal (+/+) genotype and phenotype were present in, and transmitted by, BM-derived progenitors. These data show that glomerular mesangial cell progenitors are derived from the BM and can deliver a disease phenotype to normal glomeruli. Glomerular lesions may therefore be perpetuated or aggravated, rather than resolved, by newly arriving progenitor cells exhibiting a disease phenotype. Full Text Article | | 11733560
 |