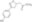475837 Sigma-AldrichMIF Antagonist, ISO-1 - CAS 478336-92-4 - Calbiochem
MIF Antagonist, ISO-1, CAS 478336-92-4, is a cell-permeable inhibitor of MIF tautomerase (IC50 = 7 µM for D-dopachrome tautomerase). Suppresses the production of TNFα, PGE2 & COX-2 in monocytes.
More>> MIF Antagonist, ISO-1, CAS 478336-92-4, is a cell-permeable inhibitor of MIF tautomerase (IC50 = 7 µM for D-dopachrome tautomerase). Suppresses the production of TNFα, PGE2 & COX-2 in monocytes. Less<<Synonyms: (S,R)-3-(4-Hydroxyphenyl)-4,5-dihydro-5-isoxazole acetic acid, methyl ester, Macrophage Migration Inhibitory Factor Antagonist, ISO-1
Recommended Products
Overview
| Replacement Information |
|---|
Key Spec Table
| CAS # | Empirical Formula |
|---|---|
| 478336-92-4 | C₁₂H₁₃NO₄ |
Products
| Catalogue Number | Packaging | Qty/Pack | |
|---|---|---|---|
| 475837-5MG | Plastic ampoule | 5 mg |
| Description | |
|---|---|
| Overview | A cell-permeable isoxazoline compound that displays anti-inflammatory properties. Inhibits MIF tautomerase activity by binding to its catalytic active site (IC50 = 7 µM for D-dopachrome tautomerase) and suppresses the production of TNFα, PGE2 and COX-2 in human monocytes, and arachidonic acid in RAW 264.7 macrophages. Shown to exhibit antidiabetogenic properties in immunoinflammatory diabetic mouse model. Also available as a 50 mM solution in DMSO (Cat. No. 505225). |
| Catalogue Number | 475837 |
| Brand Family | Calbiochem® |
| Synonyms | (S,R)-3-(4-Hydroxyphenyl)-4,5-dihydro-5-isoxazole acetic acid, methyl ester, Macrophage Migration Inhibitory Factor Antagonist, ISO-1 |
| References | |
|---|---|
| References | Cvetkovic, I., et al. 2005. Endocrinology, 146, 2942. Lubetsky, J.B., et al. 2002. J. Biol. Chem. 277, 24976. |
| Product Information | |
|---|---|
| CAS number | 478336-92-4 |
| ATP Competitive | N |
| Form | Light yellow solid |
| Hill Formula | C₁₂H₁₃NO₄ |
| Chemical formula | C₁₂H₁₃NO₄ |
| Reversible | N |
| Structure formula Image | |
| Quality Level | MQ100 |
| Biological Information | |
|---|---|
| Primary Target | MIF tautomerize |
| Primary Target IC<sub>50</sub> | 7 µM for D-dopachrome tautomerase |
| Purity | ≥98% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| 475837-5MG | 04055977184969 |
Documentation
MIF Antagonist, ISO-1 - CAS 478336-92-4 - Calbiochem SDS
| Title |
|---|
MIF Antagonist, ISO-1 - CAS 478336-92-4 - Calbiochem Certificates of Analysis
| Title | Lot Number |
|---|---|
| 475837 |
References
| Reference overview |
|---|
| Cvetkovic, I., et al. 2005. Endocrinology, 146, 2942. Lubetsky, J.B., et al. 2002. J. Biol. Chem. 277, 24976. |