Hepatic apolipoprotein M (apoM) overexpression stimulates formation of larger apoM/sphingosine 1-phosphate-enriched plasma high density lipoprotein.
Liu, M; Seo, J; Allegood, J; Bi, X; Zhu, X; Boudyguina, E; Gebre, AK; Avni, D; Shah, D; Sorci-Thomas, MG; Thomas, MJ; Shelness, GS; Spiegel, S; Parks, JS
The Journal of biological chemistry
289
2801-14
2014
Show Abstract
Apolipoprotein M (apoM), a lipocalin family member, preferentially associates with plasma HDL and binds plasma sphingosine 1-phosphate (S1P), a signaling molecule active in immune homeostasis and endothelial barrier function. ApoM overexpression in ABCA1-expressing HEK293 cells stimulated larger nascent HDL formation, compared with cells that did not express apoM; however, the in vivo role of apoM in HDL metabolism remains poorly understood. To test whether hepatic apoM overexpression increases plasma HDL size, we generated hepatocyte-specific apoM transgenic (APOM Tg) mice, which had an ∼3-5-fold increase in plasma apoM levels compared with wild-type mice. Although HDL cholesterol concentrations were similar to wild-type mice, APOM Tg mice had larger plasma HDLs enriched in apoM, cholesteryl ester, lecithin:cholesterol acyltransferase, and S1P. Despite the presence of larger plasma HDLs in APOM Tg mice, in vivo macrophage reverse cholesterol transport capacity was similar to that in wild-type mice. APOM Tg mice had an ∼5-fold increase in plasma S1P, which was predominantly associated with larger plasma HDLs. Primary hepatocytes from APOM Tg mice generated larger nascent HDLs and displayed increased sphingolipid synthesis and S1P secretion. Inhibition of ceramide synthases in hepatocytes increased cellular S1P levels but not S1P secretion, suggesting that apoM is rate-limiting in the export of hepatocyte S1P. Our data indicate that hepatocyte-specific apoM overexpression generates larger nascent HDLs and larger plasma HDLs, which preferentially bind apoM and S1P, and stimulates S1P biosynthesis for secretion. The unique apoM/S1P-enriched plasma HDL may serve to deliver S1P to extrahepatic tissues for atheroprotection and may have other as yet unidentified functions. | 24318881
 |
Functional LCAT deficiency in human apolipoprotein A-I transgenic, SR-BI knockout mice.
Lee, JY; Badeau, RM; Mulya, A; Boudyguina, E; Gebre, AK; Smith, TL; Parks, JS
Journal of lipid research
48
1052-61
2007
Show Abstract
Reduction of plasma LCAT activity has been observed in several conditions in which the size of HDL particles is increased; however, the mechanism of this reduction remains elusive. We investigated the plasma activity, mass, and in vivo catabolism of LCAT and its association with HDL particles in human apolipoprotein A-I transgenic, scavenger receptor class B type I knockout (hA-ITg SR-BI-/-) mice. Compared with hA-ITg mice, hA-ITg SR-BI-/- mice had a 4-fold higher total plasma cholesterol concentration, which occurred predominantly in 13-18 nm diameter HDL particles, a significant reduction in plasma esterified cholesterol-total cholesterol (EC/TC) ratio, and significantly lower plasma LCAT activity, suggesting a decrease in LCAT protein. However, LCAT protein in plasma, hepatic mRNA for LCAT, and in vivo turnover of 35S-radiolabeled LCAT were similar in both genotypes of mice. HDL from hA-ITg SR-BI-/- mice was enriched in sphingomyelin (SM), relative to phosphatidylcholine, and had less associated [35S]LCAT radiolabel and endogenous LCAT activity compared with HDL from hA-ITg mice. We conclude that the decreased EC/TC ratio in the plasma of hA-ITg SR-BI-/- mice is attributed to a reduction in LCAT reactivity with SM-enriched HDL particles. | 17272829
 |
Endothelial lipase is a major genetic determinant for high-density lipoprotein concentration, structure, and metabolism.
Ma, K; Cilingiroglu, M; Otvos, JD; Ballantyne, CM; Marian, AJ; Chan, L
Proceedings of the National Academy of Sciences of the United States of America
100
2748-53
2003
Show Abstract
High-density lipoprotein (HDL) protects against atherosclerosis. Endothelial lipase (EL) has been postulated to be involved in lipoprotein, and possibly HDL, metabolism, yet the evidence has been scarce and conflicting. We have inactivated EL in mice by gene targeting. EL(-/-) mice have elevated plasma and HDL cholesterol, and increased apolipoproteins A-I and E. NMR analysis reveals an abundance of large HDL particles. There is down-regulation of the transcripts for phospholipid transfer protein, but up-regulation of those for hepatic lipase and lipoprotein lipase. Plasma lecithin:cholesterol acyltransferase is unchanged despite an increase in hepatic mRNA; lecithin:cholesterol acyltransferase activity toward endogenous EL(-/-) substrate is, however, reduced by 50%. HDL clearance is decreased in EL(-/-) mice; both the structure of HDL and the presence of EL are factors that determine the rate of clearance. To determine EL's role in humans, we find a significant association between a single-nucleotide polymorphism 584C/T in the EL (LIPG) gene and HDL cholesterol in a well characterized population of 372 individuals. We conclude that EL is a major determinant of HDL concentration, structure, and metabolism in mice, and a major determinant of HDL concentration in humans. | 12601178
 |









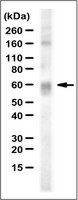

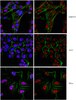




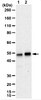
 Antibody[203635-ALL].jpg)