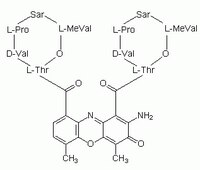
114666 Sigma-AldrichActinomycin D, Streptomyces sp. - CAS 50-76-0 - Calbiochem
Actinomycin D, Streptomyces sp., CAS 50-76-0, is an anti-neoplastic antibiotic that inhibits DNA-primed RNA polymerase by complexing with DNA via deoxyguanosine residues.
More>> Actinomycin D, Streptomyces sp., CAS 50-76-0, is an anti-neoplastic antibiotic that inhibits DNA-primed RNA polymerase by complexing with DNA via deoxyguanosine residues. Less<<Synonyms: Dactinomycin, RNA Polymerase I Inhibitor I, Pol I Inhibitor I
Recommended Products
-
575547 Sigma-Aldrich Tankyrase1/2 Inhibitor IV, JW55 - CAS 664993-53-7 - Calbiochem -

PCC030C30L Millipore Pellicon® Capsule with Ultracel® Membrane, C Screen, 3 m2 manifold with AseptiQuik® L connectors -

MABF2786-25UL Sigma-Aldrich Anti-IL-9 Antibody, clone MH9A3 -

52332-25GM Sigma-Aldrich Phenylmethylsulfonyl Fluoride - CAS 329-98-6 - Calbiochem -
820760 Sigma-Aldrich di-Manganese decacarbonyl -

KGEPS10TB1 Millipore Opticap® XL 10 Millipore Express® SHF Sterile 0.2 µm 1-1/2 in. TC-1 in. HB -

1096350005 Supelco Ethylbenzene -

102792 SAFC Copper(II) sulfate -

ZAFS0080MX Milli-Q ZAFS0080MX -

AC112C Sigma-Aldrich Sheep Anti-Human IgG Antibody, Cy3 conjugate
Overview
Key Spec Table
| CAS # | Empirical Formula |
|---|---|
| 50-76-0 | C₆₂H₈₆N₁₂O₁₆ |
| Product Information | |
|---|---|
| CAS number | 50-76-0 |
| Form | Red crystalline solid |
| Hill Formula | C₆₂H₈₆N₁₂O₁₆ |
| Chemical formula | C₆₂H₈₆N₁₂O₁₆ |
| Hygroscopic | Hygroscopic |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications | |
|---|---|
| Application | Actinomycin D, Streptomyces sp., CAS 50-76-0, is an anti-neoplastic antibiotic that inhibits DNA-primed RNA polymerase by complexing with DNA via deoxyguanosine residues. |
| Biological Information | |
|---|---|
| Primary Target | serine proteases |
| Secondary target | cell growth and colony formation in synchronized HeLa cells |
| Purity | ≥98% by HPLC |
| Safety Information according to GHS | |
|---|---|
| RTECS | AU1575000 |
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| 114666 | 0 |