574601 Sigma-AldrichSuperoxide Dismutase Assay Kit II
Empfohlene Produkte
Übersicht
| Replacement Information |
|---|
Preis & Verfügbarkeit
| Bestellnummer | Verfügbarkeit | Verpackung | St./Pkg. | Preis | Menge | |
|---|---|---|---|---|---|---|
| 574601-1KIT |
|
Glasflasche | 1 kit |
|
— |
| Product Information | |
|---|---|
| Unit of Definition | The amount of enzyme needed to exhibit 50% dismutation of the superoxide radical. |
| Detection method | Colorimetric |
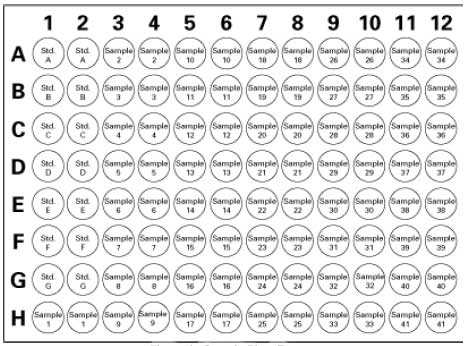
| Form | 96 Tests |
| Format | 96-well plate |
| Kit contains | Assay Buffer, Sample Buffer, Radical Detector, SOD Standard, Xanthine Oxidase, 96-Well Plate, Plate Cover, and a user protocol. |
| Positive control | Bovine erythrocyte SOD (Cu/Zn) |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
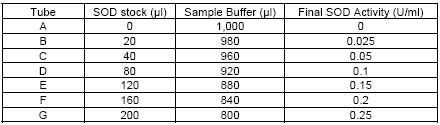
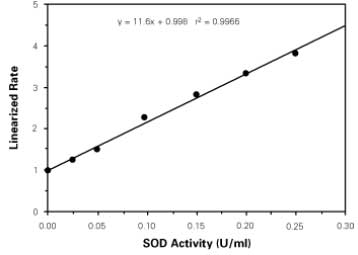
| Assay range | 0.025-0.25 units per ml SOD |
| Assay time | 1.5 h |
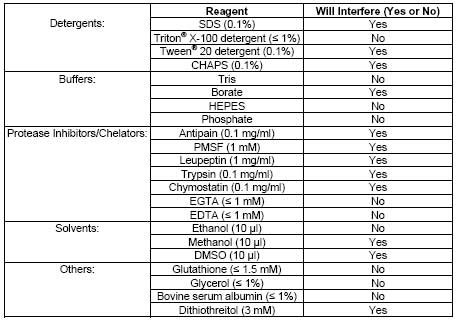
| Sample Type | Plasma, serum, erythrocyte lysates, and other lysates, tissue homogenates, and cell lysates |
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Storage and Shipping Information | |
|---|---|
| Ship Code | Dry Ice Only |
| Toxicity | Standard Handling |
| Storage | -20°C |
| Storage Conditions | Upon arrival store entire contents of the kit at -20°C. |
| Do not freeze | Ok to freeze |
| Packaging Information |
|---|
| Transport Information |
|---|
| Supplemental Information | |
|---|---|
| Kit contains | Assay Buffer, Sample Buffer, Radical Detector, SOD Standard, Xanthine Oxidase, 96-Well Plate, Plate Cover, and a user protocol. |
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Bestellnummer | GTIN |
| 574601-1KIT | 07790788051822 |
Documentation
Superoxide Dismutase Assay Kit II Analysenzertifikate
| Titel | Chargennummer |
|---|---|
| 574601 |
Literatur
| Übersicht |
|---|
| Maier, C.M. and Chan, P.H 2002. The Neuroscientist 8, 323. Mattiazz, M., et al. 2002. J. Biol. Chem. 277, 29626. Beckman, J.S. and Koppenol, W.H. 1996. Am. J. Physiol. 271, C1424. Liu, D. 1996. J. Mol. Neuro. 7, 159. MacMillan-Crow, L.A., et al. 1996. Proc. Natl. Acad. Sci. USA 93, 11853. Sun, E., et al. 1995. Biol. Trace Elem. Res. 48, 231. Sandstrom, J., et al. 1994. J. Biol. Chem. 269, 19163. Marklund, S. 1980. Acta Physiol. Scand. Suppl. 492, 19. Malstrom, B. 1975. in The Enzymes. Boyer, P., editor. XIIB, Academic Press, New York, 533. |
Broschüre
| Titel |
|---|
| Kit SourceBook - 2nd Edition EURO |