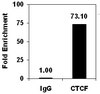
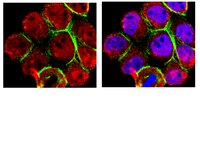
Effects of TP53 mutational status on gene expression patterns across 10 human cancer types.
Parikh, N; Hilsenbeck, S; Creighton, CJ; Dayaram, T; Shuck, R; Shinbrot, E; Xi, L; Gibbs, RA; Wheeler, DA; Donehower, LA
The Journal of pathology
232
522-33
2014
Abstract anzeigen
Mutations in the TP53 tumour suppressor gene occur in half of all human cancers, indicating its critical importance in inhibiting cancer development. Despite extensive studies, the mechanisms by which mutant p53 enhances tumour progression remain only partially understood. Here, using data from the Cancer Genome Atlas (TCGA), genomic and transcriptomic analyses were performed on 2256 tumours from 10 human cancer types. We show that tumours with TP53 mutations have altered gene expression profiles compared to tumours retaining two wild-type TP53 alleles. Among 113 known p53-up-regulated target genes identified from cell culture assays, 10 were consistently up-regulated in at least eight of 10 cancer types that retain both copies of wild-type TP53. RPS27L, CDKN1A (p21(CIP1)) and ZMAT3 were significantly up-regulated in all 10 cancer types retaining wild-type TP53. Using this p53-based expression analysis as a discovery tool, we used cell-based assays to identify five novel p53 target genes from genes consistently up-regulated in wild-type p53 cancers. Global gene expression analyses revealed that cell cycle regulatory genes and transcription factors E2F1, MYBL2 and FOXM1 were disproportionately up-regulated in many TP53 mutant cancer types. Finally, greater than 93% of tumours with a TP53 mutation exhibited greatly reduced wild-type p53 messenger expression, due to loss of heterozygosity or copy neutral loss of heterozygosity, supporting the concept of p53 as a recessive tumour suppressor. The data indicate that tumours with wild-type TP53 retain some aspects of p53-mediated growth inhibitory signalling through activation of p53 target genes and suppression of cell cycle regulatory genes. | 24374933
 |
Sulforaphane induction of p21(Cip1) cyclin-dependent kinase inhibitor expression requires p53 and Sp1 transcription factors and is p53-dependent.
Chew, YC; Adhikary, G; Wilson, GM; Xu, W; Eckert, RL
The Journal of biological chemistry
287
16168-78
2011
Abstract anzeigen
Sulforaphane (SFN) is an important cancer preventive agent derived from cruciferous vegetables. We show that SFN treatment suppresses normal human keratinocyte proliferation via a mechanism that involves increased expression of p21(Cip1). SFN treatment produces a concentration-dependent increase in p21(Cip1) promoter activity via a mechanism that involves stabilization of the p53 protein leading to increased p53 binding to the p21(Cip1) promoter p53 response elements. The proximal p21(Cip1) promoter GC-rich Sp1 factor binding elements are also required, as the SFN-dependent increase is lost when these sites are mutated. SFN treatment increases Sp1 binding to these elements, and the response is enhanced in the presence of exogenous Sp1 and reduced in the presence of ΔN-Sp3. CpG island methylation alters p21(Cip1) promoter activity some systems; however, expression in SFN-treated keratinocytes does not involve changes in proximal promoter methylation. The promoter is minimally methylated, and the methylation level is not altered by SFN treatment. This study indicates that SFN increases p21(Cip1) promoter transcription via a mechanism that involves SFN-dependent stabilization of p53 and increased p53 and Sp1 binding to their respective response elements in the p21(Cip1) promoter. These results are in marked contrast to the mechanisms observed in skin cancer cell lines and suggest that SFN may protect normal keratinocytes from damage while causing cancer cells to undergo apoptosis. | 22427654
 |