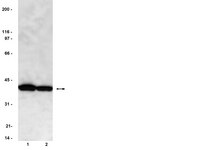
MASTL promotes cyclin B1 destruction by enforcing Cdc20-independent binding of cyclin B1 to the APC/C.
Voets, E; Wolthuis, R
Biology open
4
484-95
2015
Abstract anzeigen
When cells enter mitosis, the anaphase-promoting complex/cyclosome (APC/C) is activated by phosphorylation and binding of Cdc20. The RXXL destruction box (D-box) of cyclin B1 only binds Cdc20 after release of the spindle checkpoint in metaphase, initiating cyclin B1 ubiquitination upon chromosome bi-orientation. However, we found that cyclin B1, through Cdk1 and Cks, is targeted to the phosphorylated APC/C(Cdc20) at the start of prometaphase, when the spindle checkpoint is still active. Here, we show that MASTL is essential for cyclin B1 recruitment to the mitotic APC/C and that this occurs entirely independently of Cdc20. Importantly, MASTL-directed binding of cyclin B1 to spindle checkpoint-inhibited APC/C(Cdc20) critically supports efficient cyclin B1 destruction after checkpoint release. A high incidence of anaphase bridges observed in response to MASTL RNAi may result from cyclin B1 remaining after securin destruction, which is insufficient to keep MASTL-depleted cells in mitosis but delays the activation of separase. | | | 25750436
 |
Regulation of autophagy by coordinated action of mTORC1 and protein phosphatase 2A.
Wong, PM; Feng, Y; Wang, J; Shi, R; Jiang, X
Nature communications
6
8048
2015
Abstract anzeigen
Autophagy is a cellular catabolic process critical for cell viability and homoeostasis. Inhibition of mammalian target of rapamycin (mTOR) complex-1 (mTORC1) activates autophagy. A puzzling observation is that amino acid starvation triggers more rapid autophagy than pharmacological inhibition of mTORC1, although they both block mTORC1 activity with similar kinetics. Here we find that in addition to mTORC1 inactivation, starvation also causes an increase in phosphatase activity towards ULK1, an mTORC1 substrate whose dephosphorylation is required for autophagy induction. We identify the starvation-stimulated phosphatase for ULK1 as the PP2A-B55α complex. Treatment of cells with starvation but not mTORC1 inhibitors triggers dissociation of PP2A from its inhibitor Alpha4. Furthermore, pancreatic ductal adenocarcinoma cells, whose growth depends on high basal autophagy, possess stronger basal phosphatase activity towards ULK1 and require ULK1 for sustained anchorage-independent growth. Taken together, concurrent mTORC1 inactivation and PP2A-B55α stimulation fuel ULK1-dependent autophagy. | | | 26310906
 |
F-box protein FBXL16 binds PP2A-B55α and regulates differentiation of embryonic stem cells along the FLK1+ lineage.
Honarpour, N; Rose, CM; Brumbaugh, J; Anderson, J; Graham, RL; Sweredoski, MJ; Hess, S; Coon, JJ; Deshaies, RJ
Molecular & cellular proteomics : MCP
13
780-91
2014
Abstract anzeigen
The programmed formation of specific tissues from embryonic stem cells is a major goal of regenerative medicine. To identify points of intervention in cardiac tissue formation, we performed an siRNA screen in murine embryonic stem cells to identify ubiquitin system genes that repress cardiovascular tissue formation. Our screen uncovered an F-box protein, Fbxl16, as a repressor of one of the earliest steps in the cardiogenic lineage: FLK1+ progenitor formation. Whereas F-box proteins typically form SCF ubiquitin ligases, shotgun mass spectrometry revealed that FBXL16 instead binds protein phosphatase 2A (PP2A) containing a B55 specificity subunit (PP2A(B55)). Phosphoproteomic analyses indicate that FBXL16 negatively regulates phosphorylation of the established PP2A(B55) substrate, vimentin. We suggest that FBXL16 negatively regulates the activity of B55α-PP2A to modulate the genesis of FLK1+ progenitor cells. | Western Blotting | | 24390425
 |
Non-motor parkinsonian pathology in aging A53T α-synuclein mice is associated with progressive synucleinopathy and altered enzymatic function.
Farrell, KF; Krishnamachari, S; Villanueva, E; Lou, H; Alerte, TN; Peet, E; Drolet, RE; Perez, RG
Journal of neurochemistry
128
536-46
2014
Abstract anzeigen
Aging, the main risk factor for Parkinson's disease (PD), is associated with increased α-synuclein levels in substantia nigra pars compacta (SNc). Excess α-synuclein spurs Lewy-like pathology and dysregulates the activity of protein phosphatase 2A (PP2A). PP2A dephosphorylates many neuroproteins, including the catecholamine rate-limiting enzyme, tyrosine hydroxylase (TH). A loss of nigral dopaminergic neurons induces PD movement problems, but before those abnormalities occur, behaviors such as olfactory loss, anxiety, and constipation often manifest. Identifying mouse models with early PD behavioral changes could provide a model in which to test emerging therapeutic compounds. To this end, we evaluated mice expressing A53T mutant human (A53T) α-synuclein for behavior and α-synuclein pathology in olfactory bulb, adrenal gland, and gut. Aging A53T mice exhibited olfactory loss and anxiety that paralleled olfactory and adrenal α-synuclein aggregation. PP2A activity was also diminished in olfactory and adrenal tissues harboring insoluble α-synuclein. Low adrenal PP2A activity co-occurred with TH hyperactivity, making this the first study to link adrenal synucleinopathy to anxiety and catecholamine dysregulation. Aggregated A53T α-synuclein recombinant protein also had impaired stimulatory effects on soluble recombinant PP2A. Collectively, the data identify an excellent model in which to screen compounds for their ability to block the spread of α-synuclein pathology associated with pre-motor stages of PD. | Immunohistochemistry | | 24117685
 |
Evidence toward a dual phosphatase mechanism that restricts Aurora A (Thr-295) phosphorylation during the early embryonic cell cycle.
Kang, Q; Srividhya, J; Ipe, J; Pomerening, JR
The Journal of biological chemistry
289
17480-96
2014
Abstract anzeigen
The mitotic kinase Aurora A (AurA) is regulated by a complex network of factors that includes co-activator binding, autophosphorylation, and dephosphorylation. Dephosphorylation of AurA by PP2A (human, Ser-51; Xenopus, Ser-53) destabilizes the protein, whereas mitotic dephosphorylation of its T-loop (human, Thr-288; Xenopus, Thr-295) by PP6 represses AurA activity. However, AurA(Thr-295) phosphorylation is restricted throughout the early embryonic cell cycle, not just during M-phase, and how Thr-295 is kept dephosphorylated during interphase and whether or not this mechanism impacts the cell cycle oscillator were unknown. Titration of okadaic acid (OA) or fostriecin into Xenopus early embryonic extract revealed that phosphatase activity other than PP1 continuously suppresses AurA(Thr-295) phosphorylation during the early embryonic cell cycle. Unexpectedly, we observed that inhibiting a phosphatase activity highly sensitive to OA caused an abnormal increase in AurA(Thr-295) phosphorylation late during interphase that corresponded with delayed cyclin-dependent kinase 1 (CDK1) activation. AurA(Thr-295) phosphorylation indeed influenced this timing, because AurA isoforms retaining an intact Thr-295 residue further delayed M-phase entry. Using mathematical modeling, we determined that one phosphatase would be insufficient to restrict AurA phosphorylation and regulate CDK1 activation, whereas a dual phosphatase topology best recapitulated our experimental observations. We propose that two phosphatases target Thr-295 of AurA to prevent premature AurA activation during interphase and that phosphorylated AurA(Thr-295) acts as a competitor substrate with a CDK1-activating phosphatase in late interphase. These results suggest a novel relationship between AurA and protein phosphatases during progression throughout the early embryonic cell cycle and shed new light on potential defects caused by AurA overexpression. | | | 24825897
 |
Mutant TDP-43 deregulates AMPK activation by PP2A in ALS models.
Perera, ND; Sheean, RK; Scott, JW; Kemp, BE; Horne, MK; Turner, BJ
PloS one
9
e90449
2014
Abstract anzeigen
Bioenergetic abnormalities and metabolic dysfunctionoccur in amyotrophic lateral sclerosis (ALS) patients and genetic mouse models. However, whether metabolic dysfunction occurs earlyin ALS pathophysiology linked to different ALS genes remains unclear.Here, we investigatedAMP-activated protein kinase (AMPK) activation, which is a key enzyme induced by energy depletion and metabolic stress, inneuronal cells and mouse models expressing mutantsuperoxide dismutase 1 (SOD1)or TAR DNA binding protein 43 (TDP-43) linked to ALS.AMPKphosphorylation was sharply increased in spinal cords of transgenic SOD1G93A mice at disease onset and accumulated incytoplasmic granules in motor neurons, but not in pre-symptomatic mice. AMPK phosphorylation also occurred in peripheraltissues, liver and kidney, in SOD1G93A mice at disease onset, demonstrating that AMPK activation occurs late and is not restricted to motor neurons. Conversely, AMPK activity was drastically diminished in spinal cords and brains of presymptomatic and symptomatictransgenic TDP-43A315T mice and motor neuronal cells expressing different TDP-43 mutants. We show that mutant TDP-43 induction of the AMPK phosphatase,protein phosphatase 2A (PP2A), is associated with AMPK inactivation in these ALS models. Furthermore, PP2A inhibition by okadaic acid reversed AMPK inactivation by mutant TDP-43 in neuronal cells. Our results suggest that mutant SOD1 and TDP-43 exert contrasting effects on AMPK activation which may reflect key differences in energy metabolism and neurodegeneration in spinal cords of SOD1G93A and TDP-43A315T mice. While AMPK activation in motor neurons correlateswith progressionin mutant SOD1-mediated disease, AMPK inactivation mediated by PP2Ais associated withmutant TDP-43-linked ALS. | Western Blotting | | 24595038
 |
Cell- and virus-mediated regulation of the barrier-to-autointegration factor's phosphorylation state controls its DNA binding, dimerization, subcellular localization, and antipoxviral activity.
Jamin, A; Wicklund, A; Wiebe, MS
Journal of virology
88
5342-55
2014
Abstract anzeigen
Barrier-to-autointegration factor (BAF) is a DNA binding protein with multiple cellular functions, including the ability to act as a potent defense against vaccinia virus infection. This antiviral function involves BAF's ability to condense double-stranded DNA and subsequently prevent viral DNA replication. In recent years, it has become increasingly evident that dynamic phosphorylation involving the vaccinia virus B1 kinase and cellular enzymes is likely a key regulator of multiple BAF functions; however, the precise mechanisms are poorly understood. Here we analyzed how phosphorylation impacts BAF's DNA binding, subcellular localization, dimerization, and antipoxviral activity through the characterization of BAF phosphomimetic and unphosphorylatable mutants. Our studies demonstrate that increased phosphorylation enhances BAF's mobilization from the nucleus to the cytosol, while dephosphorylation restricts BAF to the nucleus. Phosphorylation also impairs both BAF's dimerization and its DNA binding activity. Furthermore, our studies of BAF's antiviral activity revealed that hyperphosphorylated BAF is unable to suppress viral DNA replication or virus production. Interestingly, the unphosphorylatable BAF mutant, which is capable of binding DNA but localizes predominantly to the nucleus, was also incapable of suppressing viral replication. Thus, both DNA binding and localization are important determinants of BAF's antiviral function. Finally, our examination of how phosphatases are involved in regulating BAF revealed that PP2A dephosphorylates BAF during vaccinia infection, thus counterbalancing the activity of the B1 kinase. Altogether, these data demonstrate that phosphoregulation of BAF by viral and cellular enzymes modulates this protein at multiple molecular levels, thus determining its effectiveness as an antiviral factor and likely other functions as well.The barrier-to-autointegration factor (BAF) contributes to cellular genomic integrity in multiple ways, the best characterized of which are as a host defense against cytoplasmic DNA and as a regulator of mitotic nuclear reassembly. Although dynamic phosphorylation involving both viral and cellular enzymes is likely a key regulator of multiple BAF functions, the precise mechanisms involved are poorly understood. Here we demonstrate that phosphorylation coordinately regulates BAF's DNA binding, subcellular localization, dimerization, and antipoxviral activity. Overall, our findings provide new insights into how phosphoregulation of BAF modulates this protein at multiple levels and governs its effectiveness as an antiviral factor against foreign DNA. | | | 24600006
 |
Greatwall-phosphorylated Endosulfine is both an inhibitor and a substrate of PP2A-B55 heterotrimers.
Williams, BC; Filter, JJ; Blake-Hodek, KA; Wadzinski, BE; Fuda, NJ; Shalloway, D; Goldberg, ML
eLife
3
e01695
2014
Abstract anzeigen
During M phase, Endosulfine (Endos) family proteins are phosphorylated by Greatwall kinase (Gwl), and the resultant pEndos inhibits the phosphatase PP2A-B55, which would otherwise prematurely reverse many CDK-driven phosphorylations. We show here that PP2A-B55 is the enzyme responsible for dephosphorylating pEndos during M phase exit. The kinetic parameters for PP2A-B55's action on pEndos are orders of magnitude lower than those for CDK-phosphorylated substrates, suggesting a simple model for PP2A-B55 regulation that we call inhibition by unfair competition. As the name suggests, during M phase PP2A-B55's attention is diverted to pEndos, which binds much more avidly and is dephosphorylated more slowly than other substrates. When Gwl is inactivated during the M phase-to-interphase transition, the dynamic balance changes: pEndos dephosphorylated by PP2A-B55 cannot be replaced, so the phosphatase can refocus its attention on CDK-phosphorylated substrates. This mechanism explains simultaneously how PP2A-B55 and Gwl together regulate pEndos, and how pEndos controls PP2A-B55. DOI: http://dx.doi.org/10.7554/eLife.01695.001. | | | 24618897
 |
Cytoplasmic SET induces tau hyperphosphorylation through a decrease of methylated phosphatase 2A.
Chasseigneaux, S; Clamagirand, C; Huguet, L; Gorisse-Hussonnois, L; Rose, C; Allinquant, B
BMC neuroscience
15
82
2014
Abstract anzeigen
The neuronal cytoplasmic localization of SET, an inhibitor of the phosphatase 2A (PP2A), results in tau hyperphosphorylation in the brains of Alzheimer patients through mechanisms that are still not well defined.We used primary neurons and mouse brain slices to show that SET is translocated to the cytoplasm in a manner independent of both its cleavage and over-expression. The localization of SET in the cytoplasm, either by the translocation of endogenous SET or by internalization of the recombinant full-length SET protein, induced tau hyperphosphorylation. Cytoplasmic recombinant full-length SET in mouse brain slices induced a decrease of PP2A activity through a decrease of methylated PP2A levels. The levels of methylated PP2A were negatively correlated with tau hyperphosphorylation at Ser-202 but not with the abnormal phosphorylation of tau at Ser-422.The presence of full-length SET in the neuronal cytoplasm is sufficient to impair PP2A methylation and activity, leading to tau hyperphosphorylation. In addition, our data suggest that tau hyperphosphorylation is regulated by different mechanisms at distinct sites. The translocation of SET to the neuronal cytoplasm, the low activity of PP2A, and tau hyperphosphorylation are associated in the brains of Alzheimer patients. Our data show a link between the translocation of SET in the cytoplasm and the decrease of methylated PP2A levels leading to a decrease of PP2A activity and tau hyperphosphorylation. This chain of events may contribute to the pathogenesis of Alzheimer disease. | Western Blotting | | 24981783
 |
Altered protein phosphatase 2A methylation and Tau phosphorylation in the young and aged brain of methylenetetrahydrofolate reductase (MTHFR) deficient mice.
Sontag, JM; Wasek, B; Taleski, G; Smith, J; Arning, E; Sontag, E; Bottiglieri, T
Frontiers in aging neuroscience
6
214
2014
Abstract anzeigen
Common functional polymorphisms in the methylenetetrahydrofolate reductase (MTHFR) gene, a key enzyme in folate and homocysteine metabolism, influence risk for a variety of complex disorders, including developmental, vascular, and neurological diseases. MTHFR deficiency is associated with elevation of homocysteine levels and alterations in the methylation cycle. Here, using young and aged Mthfr knockout mouse models, we show that mild MTHFR deficiency can lead to brain-region specific impairment of the methylation of Ser/Thr protein phosphatase 2A (PP2A). Relative to wild-type controls, decreased expression levels of PP2A and leucine carboxyl methyltransferase (LCMT1) were primarily observed in the hippocampus and cerebellum, and to a lesser extent in the cortex of young null Mthfr (-/-) and aged heterozygous Mthfr (+/-) mice. A marked down regulation of LCMT1 correlated with the loss of PP2A/Bα holoenzymes. Dietary folate deficiency significantly decreased LCMT1, methylated PP2A and PP2A/Bα levels in all brain regions examined from aged Mthfr (+/+) mice, and further exacerbated the regional effects of MTHFR deficiency in aged Mthfr (+/-) mice. In turn, the down regulation of PP2A/Bα was associated with enhanced phosphorylation of Tau, a neuropathological hallmark of Alzheimer's disease (AD). Our findings identify hypomethylation of PP2A enzymes, which are major CNS phosphatases, as a novel mechanism by which MTHFR deficiency and Mthfr gene-diet interactions could lead to disruption of neuronal homeostasis, and increase the risk for a variety of neuropsychiatric disorders, including age-related diseases like sporadic AD. | Western Blotting | | 25202269
 |