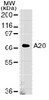
p75 neurotrophin receptor cleavage by α- and γ-secretases is required for neurotrophin-mediated proliferation of brain tumor-initiating cells.
Forsyth, PA; Krishna, N; Lawn, S; Valadez, JG; Qu, X; Fenstermacher, DA; Fournier, M; Potthast, L; Chinnaiyan, P; Gibney, GT; Zeinieh, M; Barker, PA; Carter, BD; Cooper, MK; Kenchappa, RS
The Journal of biological chemistry
289
8067-85
2014
Abstract anzeigen
Malignant gliomas are highly invasive, proliferative, and resistant to treatment. Previously, we have shown that p75 neurotrophin receptor (p75NTR) is a novel mediator of invasion of human glioma cells. However, the role of p75NTR in glioma proliferation is unknown. Here we used brain tumor-initiating cells (BTICs) and show that BTICs express neurotrophin receptors (p75NTR, TrkA, TrkB, and TrkC) and their ligands (NGF, brain-derived neurotrophic factor, and neurotrophin 3) and secrete NGF. Down-regulation of p75NTR significantly decreased proliferation of BTICs. Conversely, exogenouous NGF stimulated BTIC proliferation through α- and γ-secretase-mediated p75NTR cleavage and release of its intracellular domain (ICD). In contrast, overexpression of the p75NTR ICD induced proliferation. Interestingly, inhibition of Trk signaling blocked NGF-stimulated BTIC proliferation and p75NTR cleavage, indicating a role of Trk in p75NTR signaling. Further, blocking p75NTR cleavage attenuated Akt activation in BTICs, suggesting role of Akt in p75NTR-mediated proliferation. We also found that p75NTR, α-secretases, and the four subunits of the γ-secretase enzyme were elevated in glioblastoma multiformes patients. Importantly, the ICD of p75NTR was commonly found in malignant glioma patient specimens, suggesting that the receptor is activated and cleaved in patient tumors. These results suggest that p75NTR proteolysis is required for BTIC proliferation and is a novel potential clinical target. | 24519935
 |
Multiple mechanisms repress N-Bak mRNA translation in the healthy and apoptotic neurons.
Jakobson, M; Jakobson, M; Llano, O; Palgi, J; Arumäe, U
Cell death & disease
4
e777
2013
Abstract anzeigen
N-Bak is a neuron-specific BH3-only splice variant of pro-apoptotic Bcl-2 family member Bak. We have shown that its mRNA is stable in the neurons, whereas the protein cannot be detected by antibodies, suggesting a strong translational arrest of the mRNA. Here we identify two regulatory elements in the N-Bak mRNA that significantly repress translation in the luciferase reporter assay: an upstream open reading frame in the 5'-untranslated region (UTR) and naturally spliced exon-exon junction downstream of the premature translation termination codon in the 3'UTR. We also show that N-Bak mRNA is stored in granular structures in the sympathetic neurons and stays in these granules during intrinsic apoptosis. Finally, we confirm the absence of N-Bak protein by quantitative mass spectrometry analysis in the healthy, apoptotic or stressed sympathetic and cortical neurons. We conclude that N-Bak mRNA is translationally repressed by multiple mechanisms, and the protein does not participate in the classical apoptosis or cellular stress response. | 23969856
 |
Reduced NGF secretion by Schwann cells under the high glucose condition decreases neurite outgrowth of DRG neurons.
Takahiro Tosaki,Hideki Kamiya,Yutaka Yasuda,Keiko Naruse,Koichi Kato,Mika Kozakae,Nobuhisa Nakamura,Taiga Shibata,Yoji Hamada,Eitaro Nakashima,Yutaka Oiso,Jiro Nakamura
Experimental neurology
213
2008
Abstract anzeigen
Schwann cells (SCs) have been supposed to play prominent roles in axonal regeneration under various diseases. Here, to evaluate the direct interaction between SCs and dorsal root ganglion (DRG) neurons under a diabetic condition, the effects of Schwann cell-conditioned media on neurite outgrowth of DRG neurons were investigated. | 18675804
 |
Large-scale production of dsRNA and siRNA pools for RNA interference utilizing bacteriophage phi6 RNA-dependent RNA polymerase.
Aalto, AP; Sarin, LP; van Dijk, AA; Saarma, M; Poranen, MM; Arumäe, U; Bamford, DH
RNA (New York, N.Y.)
13
422-9
2007
Abstract anzeigen
The discovery of RNA interference (RNAi) has revolutionized biological research and has a huge potential for therapy. Since small double-stranded RNAs (dsRNAs) are required for various RNAi applications, there is a need for cost-effective methods for producing large quantities of high-quality dsRNA. We present two novel, flexible virus-based systems for the efficient production of dsRNA: (1) an in vitro system utilizing the combination of T7 RNA polymerase and RNA-dependent RNA polymerase (RdRP) of bacteriophage 6 to generate dsRNA molecules of practically unlimited length, and (2) an in vivo RNA replication system based on carrier state bacterial cells containing the 6 polymerase complex to produce virtually unlimited amounts of dsRNA of up to 4.0 kb. We show that pools of small interfering RNAs (siRNAs) derived from dsRNA produced by these systems significantly decreased the expression of a transgene (eGFP) in HeLa cells and blocked endogenous pro-apoptotic BAX expression and subsequent cell death in cultured sympathetic neurons. | 17237359
 |
Astrocytic production of nerve growth factor in motor neuron apoptosis: implications for amyotrophic lateral sclerosis.
Mariana Pehar, Patricia Cassina, Marcelo R Vargas, Raquel Castellanos, Liliana Viera, Joseph S Beckman, Alvaro G Estévez, Luis Barbeito
Journal of neurochemistry
89
464-73
2004
Abstract anzeigen
Reactive astrocytes frequently surround degenerating motor neurons in patients and transgenic animal models of amyotrophic lateral sclerosis (ALS). We report here that reactive astrocytes in the ventral spinal cord of transgenic ALS-mutant G93A superoxide dismutase (SOD) mice expressed nerve growth factor (NGF) in regions where degenerating motor neurons expressed p75 neurotrophin receptor (p75(NTR)) and were immunoreactive for nitrotyrosine. Cultured spinal cord astrocytes incubated with lipopolysaccharide (LPS) or peroxynitrite became reactive and accumulated NGF in the culture medium. Reactive astrocytes caused apoptosis of embryonic rat motor neurons plated on the top of the monolayer. Such motor neuron apoptosis could be prevented when either NGF or p75(NTR) was inhibited with blocking antibodies. In addition, nitric oxide synthase inhibitors were also protective. Exogenous NGF stimulated motor neuron apoptosis only in the presence of a low steady state concentration of nitric oxide. NGF induced apoptosis in motor neurons from p75(NTR +/+) mouse embryos but had no effect in p75(NTR -/-) knockout embryos. Culture media from reactive astrocytes as well as spinal cord lysates from symptomatic G93A SOD mice-stimulated motor neuron apoptosis, but only when incubated with exogenous nitric oxide. This effect was prevented by either NGF or p75(NTR) blocking-antibodies suggesting that it might be mediated by NGF and/or its precursor forms. Our findings show that NGF secreted by reactive astrocytes induce the death of p75-expressing motor neurons by a mechanism involving nitric oxide and peroxynitrite formation. Thus, reactive astrocytes might contribute to the progressive motor neuron degeneration characterizing ALS. | 15056289
 |
Antibodies against mouse nerve growth factor interfere in vivo with the development of avian sensory and sympathetic neurones.
Rohrer, H, et al.
Development, 103: 545-52 (1988)
1987
Abstract anzeigen
The monoclonal antibody 27/21 directed against mouse nerve growth factor (NGF) interferes in vivo with the survival of sensory dorsal root ganglion (DRG) neurones during the development of the quail embryo: the number of DRG neurones at embryonic day 11 (E11) was reduced by about 30% in embryos treated with the antibody between E3 and E11. Neurone numbers in the nodose ganglion were not affected. The effect of NGF antibodies on sympathetic neurones was assessed by determining the levels of the adrenergic marker enzyme tyrosine hydroxylase. Both total tyrosine hydroxylase activity and protein levels in sympathetic chains were reduced by about 30% in embryos treated with 27/21 antibody but not in embryos treated with a control antibody. The 27/21 antibody cross-reacts with chick NGF-like activity as shown in vitro by the ability of the antibody to partially block the survival activity of chick-embryo-fibroblast-conditioned medium for E9 chick DRG neurones. | 3246224
 |
Nerve growth factor in sympathetic ganglia and corresponding target organs of the rat: correlation with density of sympathetic innervation.
Korsching, S and Thoenen, H
Proc. Natl. Acad. Sci. U.S.A., 80: 3513-6 (1983)
1982
Abstract anzeigen
A two-site enzyme immunoassay is described which does not suffer from artifacts inherent in previous assays and has the necessary high sensitivity to determine the endogenous levels of nerve growth factor (NGF) in the sympathetic nervous system and its target organs. Monoclonal and affinity-purified polyclonal antibodies against mouse NGF (mNGF) were covalently linked to glass beads as the first site and coupled to the enzyme beta-galactosidase as the second site. Detection of the fluorescent beta-galactosidase reaction product permitted the determination of 0.01-0.02 fmol of mNGF per assay. The recovery of mNGF added to homogenates varied between 50% and 100%, depending on the tissue. Rat superior cervical and stellate ganglia were found to contain (mean +/- SEM) 25 +/- 4 and 19 +/- 3 ng of NGF per g wet weight, respectively, and the densely innervated submandibular gland, heart atrium, and iris contained 0.5 +/- 0.1, 1.0 +/- 0.1, and 1.9 +/- 0.3 ng of NGF per g wet weight, respectively. Heart ventricle and skeletal muscle, which are poorly innervated by the sympathetic nervous system, did not contain detectable levels of NGF (less than 0.3 ng/g wet weight). Serum contained less than 0.05 ng of NGF per ml. The correlation between NGF levels and density of innervation is consistent with the concept that the production of NGF in target organs determines their density of innervation by the sympathetic nervous system. | 6407016
 |
Physiology of nerve growth factor.
Thoenen, H and Barde, Y A
Physiol. Rev., 60: 1284-335 (1980)
1980
| 6159658
 |
Nerve growth factor: biological significance, measurement, and distribution.
Harper, G P and Thoenen, H
J. Neurochem., 34: 5-16 (1980)
1980
| 6161212
 |
Nerve growth factor in mouse and rat serum: correlation between bioassay and radioimmunoassay determinations.
Suda, K, et al.
Proc. Natl. Acad. Sci. U.S.A., 75: 4042-6 (1978)
1977
Abstract anzeigen
High levels of nerve growth factor (NGF) determined by competition radioimmunoassay do not agree with values obtained by bioassay. This discrepancy is illustrated here with rat and mouse serum as examples in which values up to 1000 ng/ml have been found by using competition radioimmunoassays. An explanation for the discordant results is presented: serum components bind NGF with an intermediate affinity (Kd = 10(-7) M) but with a very large capacity (up to 0.5 mg of NGF per ml of rat serum). The binding of 125I-labeled NGF to serum components competes with the binding to the solid-phase antibodies (Kd = 10(-9)M) present in limiting amounts, according to the principle of competition radioimmunoassays. Thus, less radioactivity is recovered bound to the antibodies and this gives the erroneous impression that NGF is present. To overcome this difficulty, a two-site radioimmunoassay has been developed which utilizes nonlimiting numbers of antibody binding sites. This assay provides a reliable determination of NGF levels in serum and it can be shown that in rat and mouse serum (either sex) there is less than 5 ng of NGF per ml, in agreement with the results of the bioassay. | 279023
 |