Generation of a Retinoblastoma (Rb)1-inducible dominant-negative (DN) mouse model.
Tarang, S; Doi, SM; Gurumurthy, CB; Harms, D; Quadros, R; Rocha-Sanchez, SM
Frontiers in cellular neuroscience
9
52
2015
Abstract anzeigen
Retinoblastoma 1 (Rb1) is an essential gene regulating cellular proliferation, differentiation, and homeostasis. To exert these functions, Rb1 is recruited and physically interacts with a growing variety of signaling pathways. While Rb1 does not appear to be ubiquitously expressed, its expression has been confirmed in a variety of hematopoietic and neuronal-derived cells, including the inner ear hair cells (HCs). Studies in transgenic mice demonstrate that complete germline or conditional Rb1 deletion leads to abnormal cell proliferation, followed by massive apoptosis; making it difficult to fully address Rb1's biochemical activities. To overcome these limitations, we developed a tetracycline-inducible TetO-CB-myc6-Rb1 (CBRb) mouse model to achieve transient and inducible dominant-negative (DN) inhibition of the endogenous RB1 protein. Our strategy involved fusing the Rb1 gene to the lysosomal protease pre-procathepsin B (CB), thus allowing for further routing of the DN-CBRb fusion protein and its interacting complexes for proteolytic degradation. Moreover, reversibility of the system is achieved upon suppression of doxycycline (Dox) administration. Preliminary characterization of DN-CBRb mice bred to a ubiquitous rtTA mouse line demonstrated a significant inhibition of the endogenous RB1 protein in the inner ear and in a number of other organs where RB1 is expressed. Examination of the postnatal (P) DN-CBRb mice inner ear at P10 and P28 showed the presence of supernumerary inner HCs (IHCs) in the lower turns of the cochleae, which corresponds to the described expression domain of the endogenous Rb1 gene. Selective and reversible suppression of gene expression is both an experimental tool for defining function and a potential means to medical therapy. Given the limitations associated with Rb1-null mice lethality, this model provides a valuable resource for understanding RB1 activity, relative contribution to HC regeneration and its potential therapeutic application. | | | 25755634
 |
Dysregulation of astrocyte extracellular signaling in Costello syndrome.
Krencik, R; Hokanson, KC; Narayan, AR; Dvornik, J; Rooney, GE; Rauen, KA; Weiss, LA; Rowitch, DH; Ullian, EM
Science translational medicine
7
286ra66
2015
Abstract anzeigen
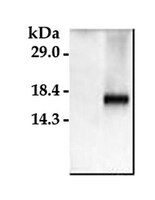
Astrocytes produce an assortment of signals that promote neuronal maturation according to a precise developmental timeline. Is this orchestrated timing and signaling altered in human neurodevelopmental disorders? To address this question, the astroglial lineage was investigated in two model systems of a developmental disorder with intellectual disability caused by mutant Harvey rat sarcoma viral oncogene homolog (HRAS) termed Costello syndrome: mutant HRAS human induced pluripotent stem cells (iPSCs) and transgenic mice. Human iPSCs derived from patients with Costello syndrome differentiated to astroglia more rapidly in vitro than those derived from wild-type cell lines with normal HRAS, exhibited hyperplasia, and also generated an abundance of extracellular matrix remodeling factors and proteoglycans. Acute treatment with a farnesyl transferase inhibitor and knockdown of the transcription factor SNAI2 reduced expression of several proteoglycans in Costello syndrome iPSC-derived astrocytes. Similarly, mice in which mutant HRAS was expressed selectively in astrocytes exhibited experience-independent increased accumulation of perineuronal net proteoglycans in cortex, as well as increased parvalbumin expression in interneurons, when compared to wild-type mice. Our data indicate that astrocytes expressing mutant HRAS dysregulate cortical maturation during development as shown by abnormal extracellular matrix remodeling and implicate excessive astrocyte-to-neuron signaling as a possible drug target for treating mental impairment and enhancing neuroplasticity. | Western Blotting | | 25947161
 |
Impact of a deletion of the full-length and short isoform of p75NTR on cholinergic innervation and the population of postmitotic doublecortin positive cells in the dentate gyrus.
Poser, R; Dokter, M; von Bohlen Und Halbach, V; Berger, SM; Busch, R; Baldus, M; Unsicker, K; von Bohlen Und Halbach, O
Frontiers in neuroanatomy
9
63
2015
Abstract anzeigen
Analyses of mice carrying a deletion of the pan-neurotrophin receptor p75NTR have allowed identifying p75NTR as an important structural regulator of the hippocampus. Most of the previous analyses were done using p75NTR (ExIII) knockout mice which still express the short isoform of p75NTR. To scrutinize the role of p75NTR in the hippocampus, we analyzed adult and aged p75NTR (ExIV) knockout mice, in which both, the short and the full-length isoform are deleted. Deletion of these isoforms induced morphological alterations in the adult dentate gyrus (DG), leading to an increase in the thickness of the molecular and granular layer. Based on these observations, we next determined the morphological substrates that might contribute to this phenotype. The cholinergic innervation of the molecular and granular layer of the DG was found to be significantly increased in the knockout mice. Furthermore, adult neurogenesis in the DG was found to be significantly altered with increased numbers of doublecortin (DCX) positive cells and reduced numbers of apoptotic cells in p75NTR (ExIV) knockout mice. However, cell proliferation as measured by phosphohiston H3 (PH3) positive cell numbers was not affected. These morphological alterations (number of DCX-positive cells and increased cholinergic fiber densities) as well as reduced cell death in the DG are likely to contribute to the observed thickening of the granular layer in p75NTR (ExIV) knockout mice. In addition, Sholl-analysis of DCX-positive neurons revealed a higher dendritic complexity and could thus be a possible morphological correlate for the increased thickness of the molecular layer in p75NTR deficient animals. Our data clearly demonstrate that deletion of both, the short and the full-length isoform of p75NTR affects DG morphology, due to alterations of the cholinergic system and an imbalance between neurogenesis and programmed cell death within the subgranular zone. | | | 26074780
 |
Neuronal activity regulates remyelination via glutamate signalling to oligodendrocyte progenitors.
Gautier, HO; Evans, KA; Volbracht, K; James, R; Sitnikov, S; Lundgaard, I; James, F; Lao-Peregrin, C; Reynolds, R; Franklin, RJ; Káradóttir, RT
Nature communications
6
8518
2015
Abstract anzeigen
Myelin regeneration can occur spontaneously in demyelinating diseases such as multiple sclerosis (MS). However, the underlying mechanisms and causes of its frequent failure remain incompletely understood. Here we show, using an in-vivo remyelination model, that demyelinated axons are electrically active and generate de novo synapses with recruited oligodendrocyte progenitor cells (OPCs), which, early after lesion induction, sense neuronal activity by expressing AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid)/kainate receptors. Blocking neuronal activity, axonal vesicular release or AMPA receptors in demyelinated lesions results in reduced remyelination. In the absence of neuronal activity there is a ∼6-fold increase in OPC number within the lesions and a reduced proportion of differentiated oligodendrocytes. These findings reveal that neuronal activity and release of glutamate instruct OPCs to differentiate into new myelinating oligodendrocytes that recover lost function. Co-localization of OPCs with the presynaptic protein VGluT2 in MS lesions implies that this mechanism may provide novel targets to therapeutically enhance remyelination. | | | 26439639
 |
Folate deficiency decreases apoptosis of endometrium decidual cells in pregnant mice via the mitochondrial pathway.
Liao, XG; Li, YL; Gao, RF; Geng, YQ; Chen, XM; Liu, XQ; Ding, YB; Mu, XY; Wang, YX; He, JL
Nutrients
7
1916-32
2015
Abstract anzeigen
It is well known that maternal folate deficiency results in adverse pregnancy outcomes. In addition to aspects in embryonic development, maternal uterine receptivity and the decidualization of stromal cells is also very important for a successful pregnancy. In this study, we focused on endometrium decidualization and investigated whether apoptosis, which is essential for decidualization, was impaired. Flow cytometry and TUNEL detection revealed that apoptosis of mouse endometrium decidual cells was suppressed in the dietary folate-deficient group on Days 7 and 8 of pregnancy (Day 1 = vaginal plug) when decidua regression is initiated. The endometrium decidual tissue of the folate deficiency group expressed less Bax compared to the normal diet group while they had nearly equal expression of Bcl2 protein. Further examination revealed that the mitochondrial transmembrane potential (ΔΨm) decreased, and the fluorescence of diffuse cytoplasmic cytochrome c protein was detected using laser confocal microscopy in normal decidual cells. However, no corresponding changes were observed in the folate-deficient group. Western blotting analyses confirmed that more cytochrome c was released from mitochondria in normal decidual cells. Taken together, these results demonstrated that folate deficiency could inhibit apoptosis of decidual cells via the mitochondrial apoptosis pathway, thereby restraining decidualization of the endometrium and further impairing pregnancy. | | | 25781218
 |
Pathological roles of the VEGF/SphK pathway in Niemann-Pick type C neurons.
Lee, H; Lee, JK; Park, MH; Hong, YR; Marti, HH; Kim, H; Okada, Y; Otsu, M; Seo, EJ; Park, JH; Bae, JH; Okino, N; He, X; Schuchman, EH; Bae, JS; Jin, HK
Nature communications
5
5514
2014
Abstract anzeigen
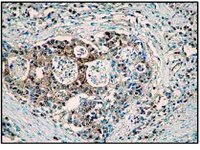
Sphingosine is a major storage compound in Niemann-Pick type C disease (NP-C), although the pathological role(s) of this accumulation have not been fully characterized. Here we found that sphingosine kinase (SphK) activity is reduced in NP-C patient fibroblasts and NP-C mouse Purkinje neurons (PNs) due to defective vascular endothelial growth factor (VEGF) levels. Sphingosine accumulation due to inactivation of VEGF/SphK pathway led to PNs loss via inhibition of autophagosome-lysosome fusion in NP-C mice. VEGF activates SphK by binding to VEGFR2, resulting in decreased sphingosine storage as well as improved PNs survival and clinical outcomes in NP-C cells and mice. We also show that induced pluripotent stem cell (iPSC)-derived human NP-C neurons are generated and the abnormalities caused by VEGF/SphK inactivity in these cells are corrected by replenishment of VEGF. Overall, these results reveal a pathogenic mechanism in NP-C neurons where defective SphK activity is due to impaired VEGF levels. | Immunofluorescence | | 25417698
 |
Inhibition of proliferation and migration of luminal and claudin-low breast cancer cells by PDGFR inhibitors.
Stalker, L; Pemberton, J; Moorehead, RA
Cancer cell international
14
89
2014
Abstract anzeigen
Platelet-derived growth factors (PDGFs) bind to two receptors, PDGFRα and PDGFRβ to mediate cell proliferation, migration and survival. Although epithelial cells typically do not express high levels of PDGFRs, their expression has been reported to increase in breast cancer cells that have undergone epithelial to mesenchymal transition.PDGFR signaling was inhibited using Sunitinib malate, Imatinib mesylate or Regorafenib in murine and human luminal-like and claudin-low mammary tumor cell lines or Masitinib in only the human cell lines. A scratch wound assay was used to assess tumor cell migration while immunofluorescence for phosphorylated histone H3 or cleaved caspase 3 was used to determine tumor cell proliferation and apoptosis, respectively.Sunitinib and Regorafenib, but not Imatinib, were capable of significantly inhibiting the migration of both murine and human luminal-like and claudin-low breast cancer cells while Masitinib inhibited migration in both human breast cancer cell lines. Sunitinib but not Regorafenib or Imatinib also significantly suppressed tumor cell proliferation in all four cell lines tested while Masitinib had no significant effect on human breast cancer cell proliferation. None of the PDGFR inhibitors consistently regulated mammary tumor cell apoptosis.Sunitinib, Regorafenib and Masitinib may prove clinically useful in inhibiting breast cancer cell migration and metastasis while only Sunitinib (and possibly Regorafenib in some breast cancer subtypes) is effective at inhibiting both migration and proliferation of breast cancer cells. | Immunofluorescence | | 25253994
 |
Novel somatic single nucleotide variants within the RNA binding protein hnRNP A1 in multiple sclerosis patients.
Lee, S; Levin, M
F1000Research
3
132
2014
Abstract anzeigen
Some somatic single nucleotide variants (SNVs) are thought to be pathogenic, leading to neurological disease. We hypothesized that heterogeneous nuclear ribonuclear protein A1 (hnRNP A1), an autoantigen associated with multiple sclerosis (MS) would contain SNVs. MS patients develop antibodies to hnRNP A1 (293-304), an epitope within the M9 domain (AA (268-305)) of hnRNP A1. M9 is hnRNP A1's nucleocytoplasmic transport domain, which binds transportin-1 (TPNO-1) and allows for hnRNP A1's transport into and out of the nucleus. Genomic DNA sequencing of M9 revealed nine novel SNVs that resulted in an amino acid substitution in MS patients that were not present in controls. SNVs occurred within the TPNO-1 binding domain (hnRNP A1 (268-289)) and the MS IgG epitope (hnRNP A1 (293-304)), within M9. In contrast to the nuclear localization of wild type (WT) hnRNP A1, mutant hnRNP A1 mis-localized to the cytoplasm, co-localized with stress granules and caused cellular apoptosis. Whilst WT hnRNP A1 bound TPNO-1, mutant hnRNP A1 showed reduced TPNO-1 binding. These data suggest SNVs in hnRNP A1 might contribute to pathogenesis of MS. | Immunofluorescence | | 25254102
 |
Erythropoietin improved cognitive function and decreased hippocampal caspase activity in rat pups after traumatic brain injury.
Schober, ME; Requena, DF; Block, B; Davis, LJ; Rodesch, C; Casper, TC; Juul, SE; Kesner, RP; Lane, RH
Journal of neurotrauma
31
358-69
2014
Abstract anzeigen
Traumatic brain injury (TBI) is a leading cause of acquired neurologic disability in children. Erythropoietin (EPO), an anti-apoptotic cytokine, improved cognitive outcome in adult rats after TBI. To our knowledge, EPO has not been studied in a developmental TBI model.We hypothesized that EPO would improve cognitive outcome and increase neuron fraction in the hippocampus in 17-day-old (P17) rat pups after controlled cortical impact (CCI).EPO or vehicle was given at 1, 24, and 48 h after CCI and at post injury day (PID) 7. Cognitive outcome at PID14 was assessed using Novel Object Recognition (NOR). Hippocampal EPO levels, caspase activity, and mRNA levels of the apoptosis factors Bcl2, Bax, Bcl-xL, and Bad were measured during the first 14 days after injury. Neuron fraction and caspase activation in CA1, CA3, and DG were studied at PID2.EPO normalized recognition memory after CCI. EPO blunted the increased hippocampal caspase activity induced by CCI at PID1, but not at PID2. EPO increased neuron fraction in CA3 at PID2. Brain levels of exogenous EPO appeared low relative to endogenous. Timing of EPO administration was associated with temporal changes in hippocampal mRNA levels of EPO and pro-apoptotic factors. Conclusion/Speculation: EPO improved recognition memory, increased regional hippocampal neuron fraction, and decreased caspase activity in P17 rats after CCI. We speculate that EPO improved cognitive outcome in rat pups after CCI as a result of improved neuronal survival via inhibition of caspase-dependent apoptosis early after injury. | | | 23972011
 |
Nuclear lamin stiffness is a barrier to 3D migration, but softness can limit survival.
Harada, T; Swift, J; Irianto, J; Shin, JW; Spinler, KR; Athirasala, A; Diegmiller, R; Dingal, PC; Ivanovska, IL; Discher, DE
The Journal of cell biology
204
669-82
2014
Abstract anzeigen
Cell migration through solid tissue often involves large contortions of the nucleus, but biological significance is largely unclear. The nucleoskeletal protein lamin-A varies both within and between cell types and was shown here to contribute to cell sorting and survival in migration through constraining micropores. Lamin-A proved rate-limiting in 3D migration of diverse human cells that ranged from glioma and adenocarcinoma lines to primary mesenchymal stem cells (MSCs). Stoichiometry of A- to B-type lamins established an activation barrier, with high lamin-A:B producing extruded nuclear shapes after migration. Because the juxtaposed A and B polymer assemblies respectively conferred viscous and elastic stiffness to the nucleus, subpopulations with different A:B levels sorted in 3D migration. However, net migration was also biphasic in lamin-A, as wild-type lamin-A levels protected against stress-induced death, whereas deep knockdown caused broad defects in stress resistance. In vivo xenografts proved consistent with A:B-based cell sorting, and intermediate A:B-enhanced tumor growth. Lamins thus impede 3D migration but also promote survival against migration-induced stresses. | | | 24567359
 |