PEGA resins
PEGA resins swell extensively in a wide range of solvents and are ideally suited for the preparation of peptide libraries, affinity purification, and on-resin enzyme assays.
Más
PEGA resins swell extensively in a wide range of solvents and are ideally suited for the preparation of peptide libraries, affinity purification, and on-resin enzyme assays. Menos
More>>
Less<<
Descripción
Especificaciones
Información para pedidos
Documentation
Productos y aplicaciones relacionados
Familias de productos
SpheriTide™ – the sustainable support for peptide synthesisSpheriTide™ resin is a novel hydrophilic, high-load support for both research and large-scale production of peptides.Más información >> |
NovaGel™ resins for peptide and organic synthesisNovaGel™ resins for peptide and organic synthesis: High substitution, broad solvent compatibility and excellent swelling properties make it an excellent support for peptide and organic synthesis.Más información >> |

NovaPEG resinsExcellent support for synthesis of long and difficult peptides. Work synergistically with pseudoproline dipeptides.Más información >> |
NovaSyn® TG resins for solid phase peptide synthesisHigh quality resins suitable for continuous flow and batch synthesis – long and challenging sequencesMás información >> |
Productos relacionados por: Brand Facete
| Novabiochem® |
Categorías
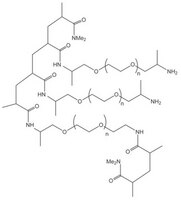
PEGA resins are hydrophilic polymers that consist of 2-acrylamidoprop-1-yl-(2-aminoprop-1-yl) polyethylene glycol800 and dimethylacylamide cross-linked with bis 2-acrylamidoprop-1-yl polyethylene glycol800 1. These supports swell extensively in a wide range of solvents, including water, DMF, DCM, THF and MeOH, and are freely permeable to macromolecules up to 35kD. These characteristics make them ideally suited for the preparation of peptide libraries, affinity purification, and on-resin enzyme assays. These properties have been exploited in the on-resin enzymatic synthesis of glycopeptides2; for determining the inhibitors of subtilisin Carlsberg3, Cruzipain4, cysteine proteases5, and matrix metalloproteinases6; and in studies on protein disulfide isomerases using fluorescence-quenched libraries7.
1. M. Meldal (1992) Tetrahedron Lett., 33, 3077.
2. M. Meldal et al. (1994) J. Chem. Soc., Chem. Commun., 1849.
3. M. Meldal et al. (1994) Proc. Natl. Acad. Sci. USA, 91, 3314.
4. M. Meldal, et al. (1998) J. Peptide Sci., 4, 83.
5. P. M. St. Hilaire, et al. (1999) J. Comb. Chem., 1, 509.
6. J. Buchardt, et al. (2000) J. Comb. Chem., 2, 624.
7. J. C. Spetzler, et al. (1998) J. Peptide Sci., 4, 128.






