344270 Sigma-AldrichForskolin, Coleus forskohlii - CAS 66575-29-9 - Calbiochem
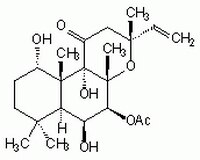
Sinónimos: 7β-Acetoxy-8,13-epoxy-1α,6β,9α-trihydroxy-labd-14-en-11-one, Colforsin
Productos recomendados
Descripción
| Replacement Information |
|---|
Tabla espec. clave
| CAS # | Empirical Formula |
|---|---|
| 66575-29-9 | C₂₂H₃₄O₇ |
Precios y disponibilidad
| Número de referencia | Disponiblidad | Embalaje | Cant./Env. | Precio | Cantidad | |
|---|---|---|---|---|---|---|
| 344270-10MG |
|
Ampolla de plást. | 10 mg |
|
— |
| Product Information | |
|---|---|
| CAS number | 66575-29-9 |
| ATP Competitive | N |
| Form | White to off-white crystalline solid |
| Hill Formula | C₂₂H₃₄O₇ |
| Chemical formula | C₂₂H₃₄O₇ |
| Reversible | N |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS | |
|---|---|
| RTECS | QL6150000 |
| Safety Information | |
|---|---|
| R Phrase | R: 21 Harmful in contact with skin. |
| S Phrase | S: 36 Wear suitable protective clothing. |
| Product Usage Statements |
|---|
| Packaging Information |
|---|
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Número de referencia | GTIN |
| 344270-10MG | 07790788049294 |
Documentation
Forskolin, Coleus forskohlii - CAS 66575-29-9 - Calbiochem Ficha datos de seguridad (MSDS)
| Título |
|---|
Forskolin, Coleus forskohlii - CAS 66575-29-9 - Calbiochem Certificados de análisis
| Cargo | Número de lote |
|---|---|
| 344270 |
Referencias bibliográficas
| Visión general referencias |
|---|
| D'Orazio, J.A., et al. 2006. Nature 443, 340. Noveen, A., et al. 1996. Biochem. Biophys. Res. Commun. 219, 180. Galli, C., et al. 1995. J. Neurosci. 15, 1172. Li, X., et al. 1995. Am. J. Physiol. 269, C986. Lomo, J., et al. 1995. J. Immunol. 154, 1634. Uneyama, H., et al. 1993. J. Biol. Chem. 268, 168. Laurenza, A., et al. 1989. Trends Pharmacol. Sci. 10, 442. Adashi, E.Y., and Resnick, C.E. 1986. J. Cell. Biochem. 31, 217. Seamon, K.B., and Daly, J.W. 1986. Adv. Cyclic Nucleotide Protein Phosphorylation Res. 20, 1. Huang, R., et al. 1982. Cyclic Nucleotide Res. 8, 385. Metzger, H., and Lindner, E. 1981. IRCS Med. Sci. Biochem. Cardiovasc. System Pharmacol. 9, 99. |
Folleto
| Cargo |
|---|
| Activators and Inhibitors of Adenylate Cyclase Technical Bulletin |
Citas
| Título | |
|---|---|
|
|
| Ficha técnica | ||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Note that this data sheet is not lot-specific and is representative of the current specifications for this product. Please consult the vial label and the certificate of analysis for information on specific lots. Also note that shipping conditions may differ from storage conditions.
|