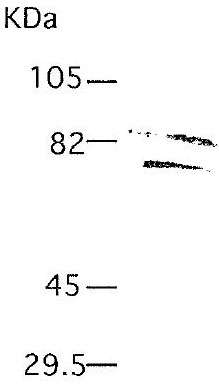
IM51 Sigma-AldrichAnti-MMP-2 (Ab-4) Mouse mAb (75-7F7)
Recommended Products
Overview
| Replacement Information |
|---|
Key Spec Table
| Species Reactivity | Host | Antibody Type |
|---|---|---|
| B, H, M | M | Monoclonal Antibody |
Pricing & Availability (Price without Taxes)
| Catalogue Number | Availability | Packaging | Qty/Pack | Price(Price without taxes) | Quantity | |
|---|---|---|---|---|---|---|
| IM51-100UG |
|
Plastic ampoule | 100 μg |
|
— |
| Product Information | |
|---|---|
| Declaration | Manufactured by Daiichi Fine Chemical Co., Ltd. Not available for sale in Japan. |
| Form | Liquid |
| Formulation | In 100 mM sodium phosphate buffer, 0.1% BSA, pH 7.0. |
| Negative control | MMP-9 protein (Cat. Nos. PF024 or PF038) |
| Positive control | MMP-2 protein (Cat. Nos. PF023 or PF037) |
| Preservative | ≤0.1% sodium azide |
| Quality Level | MQ100 |
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information |
|---|
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| IM51-100UG | 07790788053802 |
Documentation
Anti-MMP-2 (Ab-4) Mouse mAb (75-7F7) Certificates of Analysis
| Title | Lot Number |
|---|---|
| IM51 |
References
| Reference overview |
|---|
| Cottam, D. W. and Rees, R. C., 1993. Intl. J. Oncol. 2, 861. Fujimoto, N., et al. 1993. Clinica Chimica Acta. 221, 91. Stetler-Stevenson, W. G., et al. 1993. FASEB J. 7, 1434. Woessner, J. F., 1991. FASEB J. 5, 2145. Liotta, L. A. and Stetler-Stevenson, W. G., 1990. in Sem. Cancer Biol., ed. M. M. Gottesman. Vol. 1(2), 99. |