ECM550 Sigma-AldrichQCM ECMatrix Cell Invasion Assay, 24-well (8 µm), colorimetric
The CHEMICON Cell Invasion Assay Kit uses a 24-well plate, with 8 um pores, which provides an efficient system for evaluating the invasion of tumor cells through a basement membrane model.
More>> The CHEMICON Cell Invasion Assay Kit uses a 24-well plate, with 8 um pores, which provides an efficient system for evaluating the invasion of tumor cells through a basement membrane model. Less<<Recommended Products
Overview
| Replacement Information |
|---|
Key Spec Table
| Detection Methods |
|---|
| Chromogenic |
| Description | |
|---|---|
| Catalogue Number | ECM550 |
| Brand Family | Chemicon® |
| Trade Name |
|
| Description | QCM ECMatrix Cell Invasion Assay, 24-well (8 µm), colorimetric |
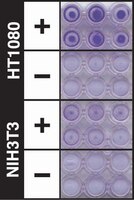
| Overview | Also available: Cell Comb™ Scratch Assay! Get biochemical data from a scratch assay! Click Here Introduction Invasion through the extracellular matrix (ECM) is an important step in tumor metastasis. Cancer cells initiate invasion by adhering to and spreading along the blood vessel wall. Proteolytic enzymes, such as MMP collagenases, dissolve tiny holes in the sheath-like covering (basement membrane) surrounding the blood vessels to allow cancer cells to invade. The CHEMICON Cell Invasion Assay Kit provides an efficient system for evaluating the invasion of tumor cells through a basement membrane model. The kit utilizes ECMatrix™, a reconstituted basement membrane matrix of proteins derived from the Engelbreth Holm-Swarm (EHS) mouse tumor. We examined the kit's performance using human fibrosarcoma (HT-1080) and non-invasive fibroblasts (NIH3T3). The CHEMICON Cell Invasion Assay Kit is ideal for evaluation of invasive tumor cells. Each CHEMICON Cell Invasion Assay Kit contains sufficient reagents for the evaluation of 12 samples. The CHEMICON Cell Invasion Assay Kit is intended for research use only; not for diagnostic or therapeutic applications. Test Principle: The CHEMICON Cell Invasion Assay is performed in an Invasion Chamber, a 24-well tissue culture plate with 12 cell culture inserts. The inserts contain an 8 μm pore size polycarbonate membrane, over which a thin layer of ECMatrixTM is dried. The ECM layer occludes the membrane pores, blocking non-invasive cells from migrating through. Invasive cells, on the other hand, migrate through the ECM layer and cling to the bottom of the polycarbonate membrane. Application: The CHEMICON Cell Invasion Assay Kit is ideal for evaluation of invasive tumor cells. Each CHEMICON Cell Invasion Assay Kit contains sufficient reagents for the evaluation of 12 samples. The CHEMICON Cell Invasion Assay Kit is intended for research use only; not for diagnostic or therapeutic applications. |
| Materials Required but Not Delivered | 1. Serum Free Media 2. Multichannel pipette and tips 3. Fetal Bovine serum 4. Tissue culture incubator 5. Microscope or Standard microplate reader 6. 10% Acetic acid 7. Beaker of Water 8. 96-well or other microtiter plate |
| References |
|---|
| Product Information | |
|---|---|
| Components |
|
| Detection method | Chromogenic |
| Quality Level | MQ100 |
| Biological Information | |
|---|---|
| Species Reactivity |
|
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Storage and Shipping Information | |
|---|---|
| Storage Conditions | Store kit materials at 2-8°C for up to 6 months. Do not freeze. |
| Packaging Information | |
|---|---|
| Material Size | 1 plate |
| Material Package | 12 wells |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| ECM550 | 04053252325052 |
Documentation
QCM ECMatrix Cell Invasion Assay, 24-well (8 µm), colorimetric SDS
| Title |
|---|
References
| Reference overview | Pub Med ID |
|---|---|
| BCAR1 protein plays important roles in carcinogenesis and predicts poor prognosis in non-small-cell lung cancer. Wei Huang,Bo Deng,Ru-Wen Wang,Qun-You Tan,Yong He,Yao-Guang Jiang,Jing-Hai Zhou PloS one 7 2012 Show Abstract | 22558353
 |
| The effect of a novel frizzled 8-related antiproliferative factor on in vitro carcinoma and melanoma cell proliferation and invasion. Kristopher R Koch,Chen-Ou Zhang,Piotr Kaczmarek,Joseph Barchi,Li Guo,Hanief M Shahjee,Susan Keay Investigational new drugs 30 2012 Show Abstract | 21931970
 |
| Epigenetic disruption of cadherin-11 in human cancer metastasis. F Javier Carmona,Alberto Villanueva,August Vidal,Clara Mu,Sara Puertas,Rosa M Penin,Montserrat Gom,Amaia Lujambio,Josep M Piulats,Ricard Mes,Montse S,Manel Man,Enric Condom,Suzanne A Eccles,Manel Esteller,Clara Muñoz,Montserrat Gomà,Ricard Mesía,Montse Sánchez-Céspedes,Manel Manós The Journal of pathology 228 2012 Show Abstract | 22374749
 |
| Combination of paclitaxel- and retinoic acid-incorporated nanoparticles for the treatment of cT-26 colon carcinoma. Hong GY, Jeong YI, Lee SJ, Lee E, Oh JS, Lee HC Archives of pharmacal research 34 407-17. Epub 2011 May 6. 2011 Show Abstract | 21547672
 |
| Down-regulation of JAK1 by RNA interference inhibits growth of the lung cancer cell line A549 and interferes with the PI3K/mTOR pathway. Dan Liu,Yi Huang,Jing Zeng,Bojiang Chen,Na Huang,Na Guo,Lunxu Liu,Hong Xu,Xianming Mo,Weimin Li Journal of cancer research and clinical oncology 137 2011 Show Abstract | 21861134
 |
| RhoBTB2 (DBC2) functions as tumor suppressor via inhibiting proliferation, preventing colony formation and inducing apoptosis in breast cancer cells. Mao H, Zhang L, Yang Y, Sun J, Deng B, Feng J, Shao Q, Feng A, Song B, Qu X Gene 486 74-80. Epub 2011 Jul 23. 2011 Show Abstract | 21801820
 |
| Mullerian Inhibiting Substance inhibits invasion and migration of epithelial cancer cell lines. Chang HL, Pieretti-Vanmarcke R, Nicolaou F, Li X, Wei X, Maclaughlin DT, Donahoe PK Gynecol Oncol 2010 Show Abstract | 21056908
 |
| The inhibitory mechanism of a novel cationic glucosamine derivative against MMP-2 and MMP-9 expressions. Eresha Mendis, Moon-Moo Kim, Niranjan Rajapakse, Se-Kwon Kim Bioorganic medicinal chemistry letters 19 2755-9 2009 Show Abstract | 19375915
 |
| Antitumor effect of all-trans retinoic acid-encapsulated nanoparticles of methoxy poly(ethylene glycol)-conjugated chitosan against CT-26 colon carcinoma in vitro. Jung-Sun Park, Yang-Seok Koh, Je-Yong Bang, Young-Il Jeong, Je-Jung Lee Journal of pharmaceutical sciences 97 4011-9 2008 Show Abstract | 18240304
 |
| Inhibition of CD44 expression in hepatocellular carcinoma cells enhances apoptosis, chemosensitivity, and reduces tumorigenesis and invasion. Zhigang Xie,Pei Feng Choong,Lai Fong Poon,Jianbiao Zhou,Jiaying Khng,Viraj Janakakumara Jasinghe,Senthilnathan Palaniyandi,Chien-Shing Chen Cancer chemotherapy and pharmacology 62 2008 Show Abstract | 18259754
 |
User Guides
| Title |
|---|
| Cell Invasion Assay Kit |