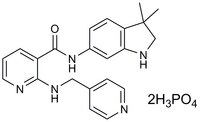
538169 Sigma-AldrichMotesanib, Diphosphate - CAS 857876-30-3 - Calbiochem
A cell-permeable, highly potent, ATP-competitive multi-kinase inhibitor (IC₅₀ = 2, 3, 6, 8 nM for VEGFR1, VEGFR2, VEGFR3, and Kit, respectively).
More>> A cell-permeable, highly potent, ATP-competitive multi-kinase inhibitor (IC₅₀ = 2, 3, 6, 8 nM for VEGFR1, VEGFR2, VEGFR3, and Kit, respectively). Less<<Synonyms: N-(3,3-dimethylindolin-6-yl)-2-((pyridin-4-ylmethyl)amino)nicotinamide, AMG-706
Recommended Products
Overview
| Replacement Information |
|---|
Key Spec Table
| CAS # | Empirical Formula |
|---|---|
| 857876-30-3 | C₂₂H₂₃N₅O.2H₃PO₄ |
Pricing & Availability
| Catalogue Number | Availability | Packaging | Qty/Pack | Price | Quantity | |
|---|---|---|---|---|---|---|
| 5.38169.0001 |
|
Glass bottle | 10 mg |
|
— |
| Product Information | |
|---|---|
| CAS number | 857876-30-3 |
| Form | Off-white solid |
| Hill Formula | C₂₂H₂₃N₅O.2H₃PO₄ |
| Chemical formula | C₂₂H₂₃N₅O.2H₃PO₄ |
| Hygroscopic | Hygroscopic |
| Reversible | Y |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | VEGFR |
| Primary Target IC<sub>50</sub> | 2 nM, 3 nM, 6 nM, 6 nM, 8 nM for VEGFR1, VEGFR2, Flk-1, VEGFR3, and KIT respecitively |
| Purity | ≥98% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| 5.38169.0001 | 04054839059414 |
Documentation
Motesanib, Diphosphate - CAS 857876-30-3 - Calbiochem SDS
| Title |
|---|
References
| Reference overview |
|---|
| Wang, Y., et al. 2014. Biochem. Pharmacol. 90, 367. Benjamin, R., et al. 2011. Cancer Chemother. Pharmacol. 68, 69. Li, C., et al. 2009. Drug Metab. Dispos. 37, 1378. Sherman, S., et al. 2008. N. Engl. J. Med. 359, 31. Polverino, A., et al. 2006. Cancer Res. 66, 8715. |