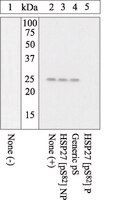
Heat shock protein 27 controls apoptosis by regulating Akt activation.
Rane, Madhavi J, et al.
J. Biol. Chem., 278: 27828-35 (2003)
2003
Show Abstract
Activation of the serine-threonine kinase Akt by cytokines, chemokines, and bacterial products delays constitutive neutrophil apoptosis, resulting in a prolonged inflammatory response. We showed previously that Akt exists in a signaling complex with p38 MAPK, MAPK-activated protein kinase-2 (MAPKAPK-2), and heat shock protein-27 (Hsp27); and Hsp27 dissociates from the complex upon neutrophil activation. To better understand the regulation of this signaling module, the hypothesis that Akt phosphorylation of Hsp27 regulates its interaction with Akt was tested. The present study shows that Akt phosphorylated Hsp27 on Ser-82 in vitro and in intact cells, and phosphorylation of Hsp27 resulted in its dissociation from Akt. Additionally, the interaction between Hsp27 and Akt was necessary for activation of Akt in intact neutrophils. Constitutive neutrophil apoptosis was accelerated by sequestration of Hsp27 from Akt, and this enhanced rate of apoptosis was reversed by introduction of constitutively active recombinant Akt. Our results define a new mechanism by which Hsp27 regulates apoptosis, through control of Akt activity. | Kinase Assay | 12740362
 |
Thyroxine pretreatment increases basal myocardial heat-shock protein 27 expression and accelerates translocation and phosphorylation of this protein upon ischaemia.
Pantos, Constantinos, et al.
Eur. J. Pharmacol., 478: 53-60 (2003)
2003
Show Abstract
Thyroxine pretreatment increases the tolerance of the heart to ischaemia, and heat-shock protein 27 (HSP27) is considered to play an important role in cardioprotection. The present study investigated whether long-term thyroxine administration can induce changes in the expression, translocation and phosphorylation of HSP27 at baseline and upon ischaemic stress. L-Thyroxine (T(4)) was administered to Wistar rats (25 microg/100 g/day s.c.) for 2 weeks, while normal animals served as controls. Hearts from normal and thyroxine-treated rats were perfused in Langendorff mode and subjected to 10 or 20 min of zero-flow global ischaemia only or to 20 min of ischaemia followed by 45 min of reperfusion. Total and phospho-HSP27 expression were assessed at different times in the Triton-soluble (cytosol-membrane), S fraction, and the Triton-insoluble (cytoskeleton-nucleus) fraction, P fraction. Postischaemic recovery of left ventricular developed pressure at 45 min of reperfusion was expressed as % of the initial value. In hearts from thyroxine-treated animals, the levels of basal total HSP27 and phospho-HSP27 in the P fraction were significantly increased as compared to normal. In response to ischaemia, in hearts from thyroxine-treated rats, the levels of total HSP27 and phospho-HSP27 were found to be significantly increased in the P fraction at 10 and 20 min of ischaemia as compared to preischaemic values, whereas in normal hearts, the levels of total HSP27 and phospho-HSP27 were significantly increased at 20 min only. Postischaemic functional recovery was significantly greater in thyroxine-treated than in untreated hearts. In summary, long-term thyroxine pretreatment results in an increased basal expression and phosphorylation of HSP27 and in an earlier and sustained redistribution of HSP27 from the S to the P fraction in response to ischaemia. This effect might be of important therapeutic relevance. | | 14555185
 |