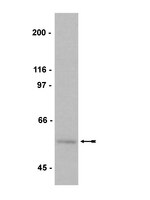
An unbiased expression screen for synaptogenic proteins identifies the LRRTM protein family as synaptic organizers.
Linhoff, MW; Laurén, J; Cassidy, RM; Dobie, FA; Takahashi, H; Nygaard, HB; Airaksinen, MS; Strittmatter, SM; Craig, AM
Neuron
61
734-49
2009
Show Abstract
Delineating the molecular basis of synapse development is crucial for understanding brain function. Cocultures of neurons with transfected fibroblasts have demonstrated the synapse-promoting activity of candidate molecules. Here, we performed an unbiased expression screen for synaptogenic proteins in the coculture assay using custom-made cDNA libraries. Reisolation of NGL-3/LRRC4B and neuroligin-2 accounts for a minority of positive clones, indicating that current understanding of mammalian synaptogenic proteins is incomplete. We identify LRRTM1 as a transmembrane protein that induces presynaptic differentiation in contacting axons. All four LRRTM family members exhibit synaptogenic activity, LRRTMs localize to excitatory synapses, and artificially induced clustering of LRRTMs mediates postsynaptic differentiation. We generate LRRTM1(-/-) mice and reveal altered distribution of the vesicular glutamate transporter VGLUT1, confirming an in vivo synaptic function. These results suggest a prevalence of LRR domain proteins in trans-synaptic signaling and provide a cellular basis for the reported linkage of LRRTM1 to handedness and schizophrenia. | | 19285470
 |
Raf-MEK-Erk cascade in anoikis is controlled by Rac1 and Cdc42 via Akt.
Zugasti, O, et al.
Mol. Cell. Biol., 21: 6706-17 (2001)
2001
Show Abstract
Signals from the extracellular matrix are essential for the survival of many cell types. Dominant-negative mutants of two members of Rho family GTPases, Rac1 and Cdc42, mimic the loss of anchorage in primary mouse fibroblasts and are potent inducers of apoptosis. This pathway of cell death requires the activation of both the p53 tumor suppressor and the extracellular signal-regulated mitogen-activated protein kinases (Erks). Here we characterize the proapoptotic Erk signal and show that it differs from the classically observed survival-promoting one by the intensity of the kinase activation. The disappearance of the GTP-bound forms of Rac1 and Cdc42 gives rise to proapoptotic, moderate activation of the Raf-MEK-Erk cascade via a signaling pathway involving the kinases phosphatidlyinositol 3-kinase and Akt. Moreover, concomitant activation of p53 and inhibition of Akt are both necessary and sufficient to signal anoikis in primary fibroblasts. Our data demonstrate that the GTPases of the Rho family control three major components of cellular signal transduction, namely, p53, Akt, and Erks, which collaborate in the induction of apoptosis due to the loss of anchorage. | Immunoprecipitation | 11533257
 |
Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product.
Evan, G I, et al.
Mol. Cell. Biol., 5: 3610-6 (1985)
1985
Show Abstract
Six monoclonal antibodies have been isolated from mice immunized with synthetic peptide immunogens whose sequences are derived from that of the human c-myc gene product. Five of these antibodies precipitate p62c-myc from human cells, and three of these five also recognize the mouse c-myc gene product. None of the antibodies sees the chicken p110gag-myc protein. All six antibodies recognize immunoblotted p62c-myc. These reagents also provide the basis for an immunoblotting assay by which to quantitate p62c-myc in cells. | | 3915782
 |