123040 Sigma-AldrichAICA-Riboside - CAS 2627-69-2 - Calbiochem
AICA-Riboside, CAS 2627-69-2, is a cell-permeable nucleoside compound whose phosphorylated metabolite activates AMPK and acts as a regulator of de novo purine synthesis.
More>> AICA-Riboside, CAS 2627-69-2, is a cell-permeable nucleoside compound whose phosphorylated metabolite activates AMPK and acts as a regulator of de novo purine synthesis. Less<<Synonyms: Acadesine, AICAr, 5-Aminoimidazole-4-carboxamide-1-β-riboside, Z-Riboside, AMPK Signaling Activator II
Recommended Products
Overview
| Replacement Information |
|---|
Key Spec Table
| CAS # | Empirical Formula |
|---|---|
| 2627-69-2 | C₉H₁₄N₄O₅ |
Pricing & Availability
| Catalogue Number | Availability | Packaging | Qty/Pack | Price | Quantity | |
|---|---|---|---|---|---|---|
| 123040-50MG |
|
Plastic ampoule | 50 mg |
|
— |
| Product Information | |
|---|---|
| CAS number | 2627-69-2 |
| ATP Competitive | N |
| Form | White to brown solid |
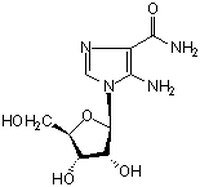
| Hill Formula | C₉H₁₄N₄O₅ |
| Chemical formula | C₉H₁₄N₄O₅ |
| Reversible | N |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | Adenosine monophosphate-activated protein kinase (AMPK) |
| Purity | ≥98% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| 123040-50MG | 04055977206272 |
Documentation
AICA-Riboside - CAS 2627-69-2 - Calbiochem Certificates of Analysis
| Title | Lot Number |
|---|---|
| 123040 |
References
| Reference overview |
|---|
| Meli, M., et al. 2006. J. Med. Chem. in press. Culmsee, C., et al. 2001. J. Mol. Neurosci. 17, 45. Muoio, D.M., et al. 1999. Biochem. J. 338, 783. Hayashi, T., et al. 1998. Diabetes 47, 1369. Corton, J.M., et al. 1995. Eur. J. Biochem. 229, 558. |