533978 Sigma-AldrichACC Inhibitor IV, CP-640186 - Calbiochem
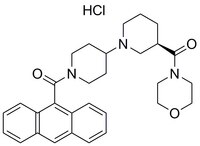
ACC Inhibitor IV, CP-640186, is a cell-permeable, potent, allosteric, eversible inhibitor of ACC (IC₅₀ = 53 and 61 nM for rat liver ACC1 & ACC2). The inhibition is uncompetitive with respect to ATP.
More>> ACC Inhibitor IV, CP-640186, is a cell-permeable, potent, allosteric, eversible inhibitor of ACC (IC₅₀ = 53 and 61 nM for rat liver ACC1 & ACC2). The inhibition is uncompetitive with respect to ATP. Less<<Synonyms: 9-Anthryl((3R)-3-(4-morpholinylcarbonyl)-1,4ʹ-bipiperidin-1ʹ-yl)methanone, HCl, Acetyl-CoA Carboxylase Inhibitor IV, CP-640186, HCl
Recommended Products
Overview
| Replacement Information |
|---|
Key Spec Table
| Empirical Formula |
|---|
| C₃₀H₃₅N₃O₃ |
Pricing & Availability
| Catalogue Number | Availability | Packaging | Qty/Pack | Price | Quantity | |
|---|---|---|---|---|---|---|
| 5.33978.0001 |
|
Glass bottle | 5 mg |
|
— |
| Product Information | |
|---|---|
| Form | Light orange solid |
| Formulation | Supplied as a hydrochloride salt. |
| Hill Formula | C₃₀H₃₅N₃O₃ |
| Chemical formula | C₃₀H₃₅N₃O₃ |
| Hygroscopic | Hygroscopic |
| Reversible | Y |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | Acetyl-CoA carboxylase (ACC) |
| Primary Target IC<sub>50</sub> | 53 nM and 61 nM for rat liver ACC1 and ACC2 |
| Purity | ≥98% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| 5.33978.0001 | 04055977281705 |
Documentation
ACC Inhibitor IV, CP-640186 - Calbiochem SDS
| Title |
|---|
ACC Inhibitor IV, CP-640186 - Calbiochem Certificates of Analysis
| Title | Lot Number |
|---|---|
| 533978 |
References
| Reference overview |
|---|
| Tong, L., and Harwood, H.J., 2006. J. Cell. Biol. 99, 1476. Gu, Y.G., et al. 2006. J. Med. Chem. 49, 3770. Zhang, H., et al. 2004. Structure 12, 1683. Harwood, H.J., et al. 2003. J. Biol. Chem. 278, 37099. |