324727 Sigma-AldrichEndoglycosidase F3, Elizabethkingia meningosepticum, Recombinant, E. coli
Endoglycosidase F3, Elizabethkingia meningosepticum, Recombinant, E. coli, cleaves asparagine-linked or free biantennary and triantennary complex, and Man3GlcNAc oligosaccharides from glycoproteins.
More>> Endoglycosidase F3, Elizabethkingia meningosepticum, Recombinant, E. coli, cleaves asparagine-linked or free biantennary and triantennary complex, and Man3GlcNAc oligosaccharides from glycoproteins. Less<<Synonyms: Endo-β-N-acetylglucosaminidase F3, Endo F3
Recommended Products
-
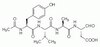
400010 Sigma-Aldrich Caspase-1 Inhibitor I - CAS 143313-51-3 - Calbiochem -

ABE40 Sigma-Aldrich Anti-PABPC1/C3 Antibody -

8920-OP Millipore OmniPur® TEMED - CAS 110-18-9 - Calbiochem -

1115865000 Supelco Potassium hydroxide solution -

CBA023 Sigma-Aldrich InnoZyme™ Cathepsin L Activity Kit, Fluorogenic -

1063499013 SAFC Sodium dihydrogen phosphate monohydrate -

HS10/4XS-N Millipore HST10 DRIVE UNIT, 0.25 KW, CE + CSA/U -

407299-20KU Sigma-Aldrich Anti-β-Interferon Rabbit pAb -

354406-1MG Sigma-Aldrich Glutaredoxin-S2, E. coli -

MAB374 Sigma-Aldrich Anti-Glyceraldehyde-3-Phosphate Dehydrogenase Antibody, clone 6C5
Overview
Pricing & Availability
| Catalogue Number | Availability | Packaging | Qty/Pack | Price | Quantity | |
|---|---|---|---|---|---|---|
| 324727-200MIU |
|
Plastic ampoule | 200 miu |
|
— |
| Description | |
|---|---|
| Overview | Recombinant, Elizabethkingia meningosepticum endoglycosidase F3 expressed in E. coli. Cleaves asparagine-linked or free biantennary and triantennary complex, and Man3GlcNAc oligosaccharides from glycoproteins. This enzyme cleaves between the two N-acetylglucosamine residues in the diacetylchitobiose core of the oligosaccharide, generating a truncated sugar molecule with one N-acetylglucosamine residue remaining on the asparagine residue. Core fucosylation increases the activity of Endo F3 up to 40 fold. Exhibits no activity on high mannose and hybrid molecules. Less sensitive to protein conformation than N-Glycosidase F (Cat. No. 362185) and therefore is more suitable for deglycosylation of native proteins. |
| Note: 1 mU = 1 milliunit. | |
| Catalogue Number | 324727 |
| Brand Family | Calbiochem® |
| Synonyms | Endo-β-N-acetylglucosaminidase F3, Endo F3 |
| Product Information | |
|---|---|
| Activity | ≥5 units/ml |
| Unit of Definition | One unit is defined as the amount of enzyme that will release N-linked oligosaccharides from 1.0 µmol porcine fibrinogen per min at 37°C, pH 5.5. |
| EC number | 3.2.1.96 |
| Form | Liquid |
| Formulation | In 20 mM Tris-HCl, pH 7.5. |
| Quality Level | MQ100 |
| Biological Information | |
|---|---|
| Specific Activity | ≥30 units/mg protein |
| Physicochemical Information | |
|---|---|
| Contaminants | Proteases: none detected |
| Storage and Shipping Information | |
|---|---|
| Ship Code | Blue Ice Only |
| Toxicity | Standard Handling |
| Storage | +2°C to +8°C |
| Do not freeze | Yes |
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| 324727-200MIU | 04055977216011 |







